
The portfolio in this agreement include approved antibiotics Merrem, Zinforo, and Zavicefta, as well as candidates in clinical development, ATM-AVI and CXL.

The portfolio in this agreement include approved antibiotics Merrem, Zinforo, and Zavicefta, as well as candidates in clinical development, ATM-AVI and CXL.

AMRI entered into a non-exclusive license agreement with the Broad Institute for the use of CRISPR-Cas9 gene-editing technology.

Steep price increases for a popular drug have created patient and Congressional backlash.

A recent paper outlines a methodology to help development teams decide whether switching from batch to continuous mode makes financial and technical sense.

Pfizer will acquire Medivation for approximately $14 billion.

The legal battle between the Broad institute and UC Berkeley heated up after an email was released from a former graduate student.

The joint venture, created through a collaboration with Bayer and CRISPR Therapeutics, will be based in Cambridge, MA.

The company is opening two offices in the United States that will offer serialization, automation, and process control services.

The company is voluntarily recalling one lot of Oxacillin for Injection, USP, 10 g.

CDMO Alcami adds HPAPI capacity and cryogenic capabilities to its Wisconsin facility.

Fresenius Kabi will add to its generic, sterile injectable manufacturing at its Melrose Park, Illinois site.

Arbor Pharmaceuticals is voluntarily recalling Cetylev (acetylcysteine) effervescent tablets due to inadequate seal of the blister pack.

TOPAS Advanced Polymers announces its COC materials are compliant with new USP standard for pharma plastic packaging systems.
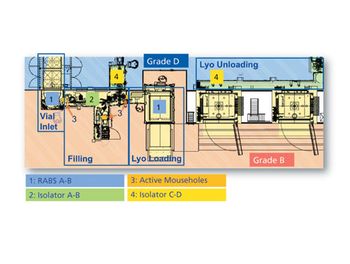
The design of Baxter BioPharma Solutions’ aseptic filling lines provides a case study in customizing containment systems for multi-product lines

The Chinese facility was cited for data integrity violations.

The Group is focusing on standardizing data exchanges between the enterprise serialization management function and product packaging lines.

Nine public health organizations submitted a letter to US House and Senate committees citing concerns about Section 11 of the FDA and NIH Workforce Authorities Modernization Act.

The bio-incubator, located at the James Cook University Hospital, will give early-stage biotechnology and life-sciences companies access to facilities to support biomedical research.

Flawed testing and analysis and a failure to analyze the root cause of customer complaints were among the top problems noted in FDA's letter to the Florida-based transdermal drug manufacturer.

The agency publishes guidance on the appropriate classification of co-crystal solid-state forms.

The company received breakthrough therapy designation for esketamine, a treatment for major depressive disorder with imminent risk for suicide.

Piramal Enterprises has entered into an agreement to acquire 100% stake in Ash Stevens all by cash for a consideration of $42.95 million plus an earn-out consideration capped at $10 million.

Irvine Scientific’s new product range includes chemically-defined, serum-free media, to increase productivity of viral vectors and recombinant proteins in suspension cultures.

A KPMG survey reveals that approximately 84% of pharma and medical device executives plan to add jobs in next 12 months.

Agilent Technologies announces plans to build a new oligo manufacturing facility in Colorado that will double current capacity.

CPhI Pharma Awards’ panel doubles in size and welcomes experts from around the world.

Mallinckrodt announced a planned merger agreement with Stratatech Corporation, a regenerative medicine company.

The agency has adopted guidelines on the pharmacovigilance of biological drugs.

A new study reveals a potential new approach to treat sickle cell disease and beta thalassemia using CRISPR-Cas9 gene-editing technology.

Jacobs Engineering will provide engineering and construction services to expand the Novartis site in Huningue, France.