
Beckman Coulter Life Science’s new Vi-Cell Metaflex is designed to be operational for more than 22 hours a day.

Beckman Coulter Life Science’s new Vi-Cell Metaflex is designed to be operational for more than 22 hours a day.

The Opadry QX from Colorcon is suitable for tablets containing temperature-sensitive active ingredients.

Plasticell was recognized for its contribution to the advancement of regenerative medicine, cell and gene therapy, as well as other areas of biomedical research.

The Subcommittee for Advanced Manufacturing of the National Science and Technology Council highlights biopharmaceutical manufacturing as an emerging priority.
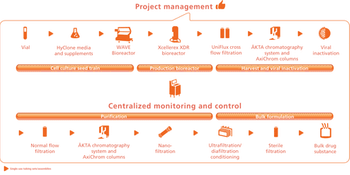
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

Pfizer announced the launch of Pfizer CentreOne, a CMO formed by the combination of Pfizer CentreSource and Hospira One 2 One.

BÜCHI Rotavapor R-300 received the German Design Awards 2016, the RedDot Award 21, and the iF International Forum Design Award.

The National Institutes of Health (NIH) released a statement on April 19, 2016 saying that it plans to suspend research in two laboratories after preliminary results found the labs not in compliance with CGMP practices.

Traditional methods of measuring the effectiveness of vaccines against the flu are called into question by new findings from the NIH.

Extract Technology’s portfolio will now include aseptic solutions, containment solutions, restricted access barriers, and mobile cleanrooms for the pharmaceutical, healthcare, biotech and chemical markets.

The guidance discusses the implementation of CMC postapproval changes through a comparability protocol.

PRAC said it has plans to review both canagliflozin and several direct-acting antivirals, after new study data showed potential risks associated with the drugs.

Sanofi will invest in its Geel, Belgium facility in order to support its pipeline of monoclonal antibodies.

The facility located in Ware, UK, will be used to manufacture GSK’s Ellipta inhaler.

Shire announces plans to build a flexible biopharmaceutical manufacturing facility in County Meath, Ireland.

Through two acquisitions, Recipharm will acquire Kemwell’s United States, Swedish, and Indian operations.

The new affiliate company will be based in Somerset, New Jersey.

The agency published guidance on data integrity as it is relates to CGMP compliance.

Linker technology and drug combinations play an important role in the efficacy of ADCs.

The National Institute of Health will conduct an internal review of the National Cancer Institute’s cell manufacturing facilities, which will affect multiple Kite projects.

FDA explains the rewards that may be associated with switching from batch to continuous manufacturing.

The agency published draft guidance on prescription requirements, hospital pharmacies, and outsourcing facilities for compounders.

The company has recalled one lot of 50% magnesium sulfate injection, USP due to particulates.

The IMS Institute for Healthcare Informatics report examines spending on medicine in the US, drivers of growth, major market segments, prescription volume, patient costs, and healthcare delivery changes.

The agencies detail the results of their three-year collaboration.

The Office of Generic Drugs highlights the agency’s work to advance generic drugs.

The valve received the highest cycle rating after completing the test at three times the required test pressure.

By focusing on laying strong technology foundations, pharmaceutical businesses can make informed decisions, equip themselves with the capabilities to eliminate threats, and future-proof operations.

JW Biotechnology will focus on developing novel cell-based immunotherapies.

The new system will be used for multiparticulate dosages.