
The conference will take place from May 16–18, 2017 in Philadelphia, Pennsylvania.

GE Healthcare Life Sciences has expanded production capabilities at its Pasching, Austria facility for sterile liquids.

FDA approved an update in the manufacturing of Prezista (darunavir) using a continuous manufacturing line at Janssen Supply Chain’s facility in Puerto Rico.

The companies will collaborate on two CSCS supply chain security studies.

The agency issues safety guidance to minimize medication errors relating to product design and container closure design.

Cornell researchers reveal that an existing FDA-approved drug can facilitate the delivery of other large molecules across the blood-brain barrier.

The Ethiopian government said it is offering tax and loan incentives to encourage local pharmaceutical production in the country

The American College of Rheumatology released a statement encouraging FDA to apply distinct names to biosimilars and maximize clarity in labels.

Sanofi says the program will vaccinate one million students from 6000 public schools in dengue-epidemic regions of the Philippines.

The FDA guidance describes information to provide to the agency when submitting a proposed proprietary name.

FDA added warning labels to medications containing saxagliptin and alogliptin after clinical trials linked the drugs to increased risk of hospitalization for heart failure.

WellSpring Pharma formed a strategic partnership with IDT Australia and invested $3 million in new equipment.

A report by Reuters found that four of the top 10 most widely used drugs increased 100% since 2011, while the other six increased 60%.

Pharma companies must weigh cost efficiencies of global API sourcing with increased complexities of monitoring for compliance and quality.

Inflectra is the second biosimilar to hit the market in the United States.

The companies announced that they have mutually decided to terminate the planned merger after the US Department of Treasury and the IRS issue temporary and proposed regulations on tax inversion.

The agency has announced the creation of the Combination Products Policy Council to address issues related to combination products.

The agency released a draft reflection paper on the extrapolation of data from adults to children in the development of pediatric drugs.

Aesica adds development capability at Queenborough with the opening of a new purpose-built facility.

EMVO appointed Andreas Walter general manager effective April 2016.

Jack Lew, Obama’s secretary of the treasury, announced on April 4, 2016, that the US Department of the Treasury and the Internal Revenue Service (IRS) is issuing temporary and proposed regulations to limit the “benefits of and limit the number of corporate tax inversions.” The government bodies also plan to address earnings stripping in these inversions, so it will examine past inversion deals that have already been completed.

Kollicoat MAE 100-55 from BASF can be used as a direct substitute in commercial enteric pharmaceutical formulations.

The company received a positive recommendation for it’s Remicade biosimilar, Flixabi (infliximab).

Sandoz Industrial Products GmbH will be renamed Corden BioChem and will be associated with the CordenPharma Group, ICIG’s pharmaceutical platform.

As part of the partnership, Zenith Technologies’ methodologies, systems, and libraries will be offered as a service to GE Healthcare’s clients in bioprocessing.

The company announced that it received EXCiPACT certification in December 2015.
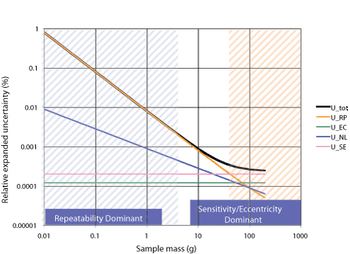
A program for calibration and routine testing of weighing instruments ensures accurate results.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss requirements for successful product technology transfer.

A global API marketplace increases the burden of supply chain monitoring for drug companies.

The companies will merge to create a gene therapy biologics CDMO.