
Regular removal of residues from disinfectants and sporicidals is important for improved aesthetics and safety in cleanrooms.

Regular removal of residues from disinfectants and sporicidals is important for improved aesthetics and safety in cleanrooms.

The directorate is looking for experts to join the European Pharmacopoeia network.

The agency published guidance on immunogenicity-related considerations for low molecular weight heparin.

The company issues a voluntary recall of a lot of 0.9% sodium chloride solution due to particulate matter.

Recipharm plans to invest €40 million in the project as a result of EMA decision to make serialization of licensed drug products a legal requirement by 2019.

The course, intended for healthcare professionals, provides an overview of biosimilar products and FDA’s biosimilar product development programs.

Unchained Labs acquires Freeslate, marking the company’s fourth acquisition in the past 12 months.

Smart glasses enhance remote troubleshooting and process management for pharmaceutical manufacturing.
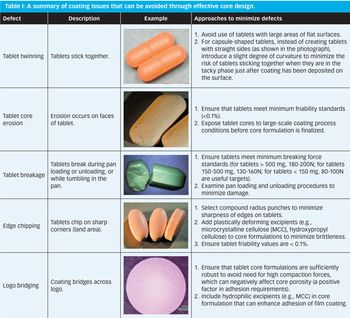
Effective design of the tablet core and the coating process can help prevent film coating problems.

The agency is continuing CDER’s Regulatory Project Management Site Tours and Regulatory Interaction Program.

This marks Vetter’s fourth internationally recognized award for its syringe closure system, Vetter-Ject.

The collaboration will allow the companies to develop and manufacture novel inhaled therapeutics.

UK pharmaceutical companies face £45 million in fines after entering pay-for-delay agreements for generic versions of paroxetine.

FDA fast-tracked the monoclonal antibody based on early clinical data from a Phase I trial.

Smart glasses enhance remote troubleshooting and process management for pharmaceutical manufacturing.

Growth in finished dosage formulations triggers a new CPhI Worldwide event.

Wyeth reaches agreement in principle to resolve Medicaid drug rebate claims for 2001-2006 period for Protonix.

Sartorius announced the release of the company’s new Tacta pipettes.

Sandoz reveals plans to complete a Phase III development program for Pfizer’s infliximab and file for registration in the EU.

FDA cracks down on makers of products extracted from Cannabis and says the products cannot be considered dietary supplements.

Multiple data-integrity violations results in FDA warning letter for Mumbai, India-based Ipca Laboratories.

MPI Research plans to renovate more than 55,000 square feet of facility space as part of the company’s plan to invest in upgrades and department expansion.

GRAM completed a fourth FDA inspection of its parenteral manufacturing facility with no Form 483 observation issues.

Vaccine R&D has grown exponentially in recent years, spurred by ethical and medical needs to combat lethal infectious outbreaks and increased funding from public and private agencies and organizations.

Biocorp’s Easylog smart sensor for insulin delivery devices enhances compliance.

Innovations awarded at Pharmapack Europe include improvements for patient compliance and protection from errors, tampering, or misuse.

Ashland Chemical’s Calvert City, KY, facility is the first in the US to receive EXCiPACT certification for GMPs of pharmaceutical excipients.

There was scant praise from the medical community for the eighth and final budget plan from the Obama administration.

Metrohm USA awards the 2016 Young Chemist Award to PhD candidate developing wearable sensors that can non-invasively monitor physiologically relevant chemicals.

3M Drug Delivery Systems, Ei Solutionworks, IDT Biologika join CMO/CDMO association