
Clinical trials of Eli Lilly’s ixekizumab showed the drug displayed clinically meaningful improvements in patients with moderate-to-severe plaque psoriasis.

Clinical trials of Eli Lilly’s ixekizumab showed the drug displayed clinically meaningful improvements in patients with moderate-to-severe plaque psoriasis.

The inspection confirmed that the facility was compliant with GMP guidelines.

The researchers examine the top 20 cancer drugs dosed by body size in the US, and estimate that drug companies will earn $1.8 billion in 2016 in revenue from leftover cancer drugs.
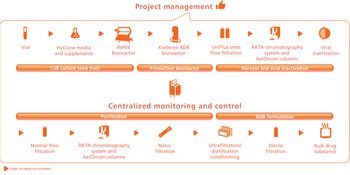
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how the regulatory requirements for CGMPs is the different phases of drug development and manufacture.

Thought leaders tackle drug shortages and manufacturing innovations.

The US Drug Enforcement Agency (DEA) has granted permission for the import of pharmaceutical-grade cannabidiol to Catalent Pharma Solutions for a feasibility study in conjunction with Kannalife Sciences, according to a company press release.

TxCell’s new facilities are based in Sophia Antipolis, France, on the premises of GenBiotech.

This marks the first time a biologic manufactured in China has been released for use in a clinical trial in Europe.

The pharma company revealed in a fourth quarter call that it will improve its cell-culture capabilities by focusing on the use of naïve, highly proliferative cells to manufacture its CAR-T drug candidate.

The new group is made up of over 15 scientists who formerly worked in GSK's computational biology and genetics departments

A reformulated version of Rose Bengal, PV-10, may be used to treat melanoma when injected directly into tumors.

CPhI Russia 2016 planned for companies seeking pharma business opportunities in region.

The program is intended to provide support to ongoing efforts in rare disease product development.

The Eppendorf and Science Prize for Neurobiology is a research prize of $25,000, given to young scientists for their contributions in neurobiology.

The agency has revised its good pharmacovigilance practices guide on risk management systems.

Company representatives said a bulk of the increase was due to strong sales of its bioprocessing products.

Cirrus Pharmaceuticals will launch services for manufacturing cGMP materials for early-phase clinical trials at its Raleigh-Durham, NC facility.

The collaboration is part of Innovate UK’s competition for the development of regenerative medicines.

Under the agreement, Caribou has granted IDT worldwide rights to commercialize CRISPR-Cas9 reagents under Caribou’s intellectual property.

Robert Califf has been confirmed by the US Senate as FDA commissioner.

ICIG’s CordenPharma Group expands fermentation-based production technology with acquisition of Sandoz Site.

Curida entered into a partnership with René Bommer, PhD, founder and owner of pharmAccel Consulting.

The lawsuit alleges EnzymeWorks and its founder engaged in willful patent infringement, misappropriation of trade secrets, and breach of confidence, in addition to other claims.

Recipharm acquires Mitim, adding scale and technology in injectable beta lactams.

Patheon will assist in the development of INTAC, a technology designed to reduce the abuse of controlled substances.

Researchers discovered more than 300 antibodies that reacted with the Ebola virus’s surface glycoprotein and could be used to neutralize many strains of the virus.

Sandoz filed a petition with the US Supreme Court to review a Federal Circuit Court’s July 2015 judgment of the Biologics Price Competition and Innovation Act (BPCIA). The pharmaceutical company filed the writ of certiorari with the court on Feb.

AAIPharma/Cambridge Major Labs plans to invest $10.7 million to relocate the company’s analytical testing facility to the Cortex Innovation Center in Missouri.

PharmSource report addresses how the opportunity for antibody drug conjugates measures up to the bio/pharma industry’s expectations.