
Pfizer names executive leadership team for combined organization upon close of proposed Allergan transaction.

Pfizer names executive leadership team for combined organization upon close of proposed Allergan transaction.

There are no clinically meaningful differences between Celltrion’s CT-P13 and Remicade, according to an FDA briefing released ahead of the formal panel meeting.

The agency prepares a plan to implement new packaging safety features.

Researchers describe a new method to compare the higher-order structure of a reference biologic with its proposed biosimilar product candidates.

FDA granted Immunomedics breakthrough therapy designation for the company’s investigational antibody drug conjugate for treatment of triple negative breast cancer.

Stephen Ostroff published a blog on FDA regarding goals to modernize the generic drug review process in an effort to increase patient access to generics.

Barvarian Nordic, Evaxion Biotech, and the Technical University of Denmark announced plans to collaborate on the development of a vaccine for MRSA.

FDA officials said on Feb. 5, 2016 that Celltrion’s biosimilar to infliximab was “highly similar” to Johnson & Johnson’s (J&J) Remicade, according to a report by Reuters.

AMETEK announced the acquisition of Brookfield Engineering Laboratories, a Massachusetts based manufacturer of viscometers and rheometers.

Although switching has occurred in European markets for some biosimilars, most biosimilar manufacturers will focus on securing new users, according to Merck.

Sartorius releases financial results for 2015, announcing a 16% increase in group sales revenue.

ViaCyte and Janssen Biotech have entered Phase I/Phase II clinical trials for VC-01, a candidate treatment for the treatment of type 1 diabetes.

In a hearing held on Feb. 4, 2016, executives from Valeant and Turing had a hard time explaining their rationales for exorbitant price hikes of older drugs.

LabConnect built a biorepository facility in Tennessee.

AstraZeneca received conditional marketing authorization for Tagrisso, a tablet for the treatment of adults with locally advanced or metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer.
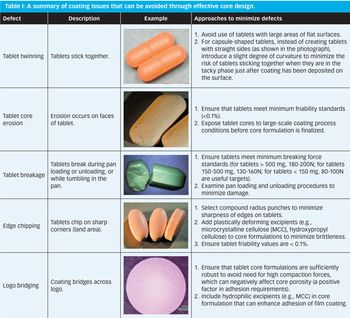
Common defects in tablet film coating can be minimized by effective design of the tablet core and the coating process.

Catalent Pharma Solutions’ technical project manager, Elanor Pinto-Cocozza, PhD, will present at InformEx 2016.

Systag, a Swiss fine chemicals and process automation company, appeared at InformEx 2016

InformEx, a conference for the fine and specialty chemicals industry, kicked off on Feb. 2, 2016 in New Orleans.

Several biopharmaceutical companies announce plans to launch R&D into Zika vaccine candidates.

The International Society for Pharmaceutical Engineering (ISPE) Facility of the Year Awards (FOYA) program announced its 2016 Category Award winners for operational excellence, sustainability, process innovation, project execution, equipment innovation, and facility integration.

Suppliers must develop new technologies to drive the bio/pharma innovation engine.

Catalent plans a $4.6 million investment to expand secondary packaging and storage in Asia.

The chemical distribution industry has formed an international chemical trade association to address global issues.

Can the feds negotiate Medicare Part D prices?

The Biosimilars Forum launched Partnership for Biosimilars Education and Access, an education initiative raising awareness of biosimilars in the US.

Pfizer and Bristol-Myers Squibb enter into agreement with Portola to develop and commercialize andexanet alfa in Japan.

CDER’s Office of Pharmaceutical Quality plans on promoting modernization as a way to ensure drug quality.

Aging populations and increased access to healthcare translates into opportunities for biopharmaceutical companies.

FDA and industry seek a more consistent, flexible CMC review process for breakthrough therapies.