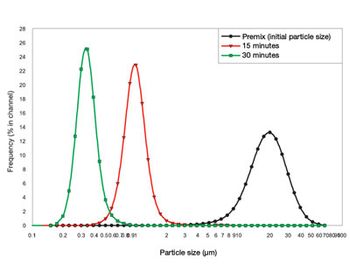
Creating nanoparticles can be challenging and requires appropriate equipment and techniques. Media milling or grinding is the best-established manufacturing method for nanoparticle production.

Creating nanoparticles can be challenging and requires appropriate equipment and techniques. Media milling or grinding is the best-established manufacturing method for nanoparticle production.

A pharmaceutical company's information-technology infrastructure could facilitate the appraisal of the performance of contract manufacturing organizations.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the July 2008 edition from Parker Fluid Control Division and Watson-Marlow Bredel.

Pfizer has agreed to pay $975,000 in civil penalties to resolve alleged violations of the Clean Air Act at its former manufacturing plant in Groton, Connecticut, according to a release by the US Department of Justice.

Also, Catalent Pharma Solutions to collaborate with One World Design and Manufacturing Group, Bioheart appoints Howard J. Leonhardt as CEO, more...

The US Food and Drug Administration is seeking volunteers from the pharmaceutical industry to participate in a pilot program involving the submission of quality information for biotechnology products.

The European Pharmacopeia Commission has published the General Information chapter "Potentially Genotoxic Impurities and European Pharmacopoeia Monographs on Substances for Human Use" in the July 2008 edition of PharmEuropa.

The European Pharmacopoeia Commission adopted revised monographs for heparin calcium and heparin sodium to strengthen the level of testing required for quality control.

Change control in life-sciences organizations is a critical business issue in terms of risk, safety, and performance. The author examines common shortcomings in change control when implementing non-enterprise solutions and the functionality derived from enterprise-level change control.

A recent US government study shows that follow-on biologics are likely to have a favorable economic impact if proposed legislation authorizing a regulatory pathway in the US is approved.

Also, Covance and WuXi PharmaTech to form contract research joint venture in China, Covidien makes appointments to its Pharmaceutical Products and Imaging Solutions businesses, more...

Richard E. Maleczka Jr. and Milton R. Smith III, professors of the Department of Chemistry at Michigan State University, were recognized by the Environmental Protection Agency's Presidential Green Chemistry Challenge Awards, for designing a catalytic reaction to synthesize precursors for the Suzuki-coupling reaction.

Molecular Profiles created a poster that demonstrates the efficacy of its "nanoPASS" (nanoscale predictive analytical screening solution) technique to characterize an active pharmaceutical ingredient's (API) surface energy.

After a one-year delay in its implementation, the US Pharmacopeia Chapter 467 "Residual Solvents" is now official.

The Congressional Budget Office has released a report that provides a picture of the financial impact from the enaction of S.1695, the Biologics Price Competition and Innovation Act of 2007.

President Bush signed H.R. 2642, the Supplemental Appropriations Act of 2008, which provides $150 million to the US Food and Drug Administration for medical-safety and drug-safety activities.
Drugmakers seeking to block the activity of a protein may have a new strategy at their disposal.

Staff reductions are not surprising with the state of the economy and falling pharmaceutical sales.

This review article explains how self-emulsifying drug delivery systems can increase the solubility and bioavailability of poorly soluble drug.

A new book explains process analytical technology, drug stability, and quality.

The year 2011 may seem far off, but there is much to do to prepare for electronic pedigrees.

A growing preference for public-private partnerships is spurring innovation and technology among Dutch pharmaceutical companies.

Clear labels for substances that can be used as excipients, APIs, or both are critical to end-product use.

They may have seemed harmless at first, but these ideas took a big bite.

With no economic relief in sight, industry, like all of us, is grappling with high-and new-costs.
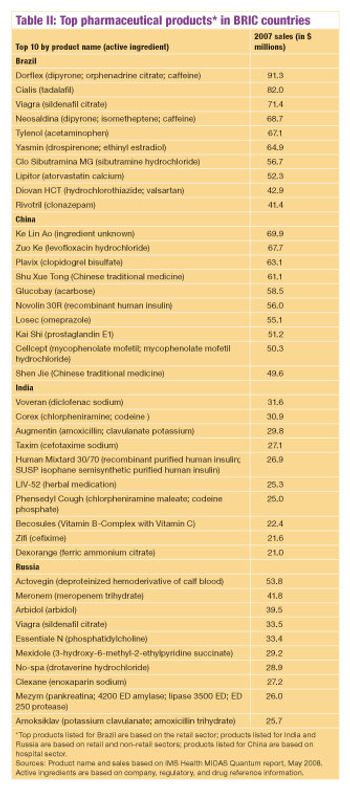
Recent reports discuss the future of pharma's emerging markets.

As summer approaches, the European pharmaceutical industry is preparing for a well-deserved break. However, some will leave the arena on a higher note than others. While big pharma is embracing the globalization of the industry and suffering under extreme pressure to improve performance, the biopharmaceutical sector retires with a smile and reasons to celebrate.

The US Pharmacopeia (USP) posted revised monographs for heparin sodium and heparin calcium on its website.

Biomedical researchers at UT Southwestern Medical Center and nanotechnology scientists at UT Dallas are collaborating on a study that seeks to selectively kill cancer cells using monoclonal antibodies to coat carbon nanotubes that heat up when exposed to near-infrared (NIR) light.

Also, Stiefel Laboratories will acquire Barrier Therapeutics, Danube Pharmaceuticals appoints Brian Levy COO, more...