
Pfizer and UCB formed a technology company named Cyclofluidic with the aim of accelerating the drug-discovery process.

Pfizer and UCB formed a technology company named Cyclofluidic with the aim of accelerating the drug-discovery process.

The US Food and Drug Administration released a draft guidance that reviews the agency's plan to offer priority-review vouchers to companies developing new treatments for neglected tropical diseases.

The US Department of Health and Human Services (HHS) will send the first Food and Drug Administration staff to China, India, Europe, and Latin America before the end of 2008.

Rep. John D. Dingell (D-MI), chairman of the US House of Representatives Committee on Energy and Commerce, and Rep. Bart Stupak (D-MI), chairman of the Oversight and Investigations Subcommittee, sent letters to the US Food and Drug Administration, Shaw Science Partners, and EthicAd to request information about a new FDA website.

Also, MedImmune opens Cambridge, UK, facility and makes reverse engineering pact with Omninvest; BD Medicine appoints Carol Adiletto VP of clinical and regulatory affairs; more...

Also, Millipore opens new membrane-casting manufacturing facility in Ireland; Surface Logix appoints Keith Dionne president, CEO, and a member of the board; more...

According to a July 11, 2008 memorandum posted by the Center for Biologics Evaluation and Research, starting Oct. 15, Health Level 7 structured product labeling in XML (extensible markup language) will be the only acceptable presentation in electronic format for the submission of content of labeling that CBER can process, review, and archive.

FDA has completed its labeling updates to fluoroquinolone antimicrobial drugs.

During the past several months, Pharmaceutical Technology has been covering the US Food and Drug Administration's rulemaking on over-the-counter (OTC) cough and cold medications for children.

At a public meeting, the Medicare Payment Advisory Commission (MedPAC) discussed its recommendation that Congress establish a national database to publicly reveal financial relationships between physicians and the pharmaceutical industry.

John Dingell (D-MI), chairman of the US House Committee on Energy and Commerce and Bart Stupak (D-MI), chairman of that committee's Subcomittee on Oversight and Investigations, directed a letter to US Food and Drug Administration Commissioner Andrew C. von Eschenbach to request further information regarding FDA's process for inspecting manufacturing facilities of the generic drug manufacturer Actavis following several product recalls by the company.

The lack of appropriate financial resources for the US Food and Drug Administration's growing global agenda has been a pertinent topic of late?and now appropriate staffing has become a large concern.

Integrating MES with enterprise resource planning (ERP) systems can bring companies greater control over production than before and make manufacturing processes more flexible.

A bill that would require country-of-origin labeling for active and inactive ingredients for all prescription and over-the-counter pharmaceuticals was introduced in the US Senate last week.

The US Food and Drug Administration?s Center for Drug Evaluation and Research (CDER) held a public meeting on Oct. 2 to discuss over-the-counter (OTC) cough and cold medications for pediatric use.

Under a definitive merger agreement, Eli Lilly and Company will acquire ImClone Systems, Inc. (New York) in a cash tender offer of approximately $6.5 billion.

Rep. John D. Dingell (D-MI), chairman of the US House of Representatives Committee on Energy and Commerce, and Rep. Bart Stupak (D-MI), the chairman of the Oversight and Investigations Subcommittee, sent a letter to the US Department of Health and Human Services (HHS), questioning the US Food and Drug Administration?s use of agency resources to hire an outside public-relations firm to create a positive public image of the agency.

The electronic pedigree mandate for prescription drugs has been delayed again. California Gov. Arnold Schwarzenegger signed legislation that delays ePedigree implementation until 2015.

Also, Merck & Co. discontinues development of its obesity drug taranabant; Synthetech names Frederic Farkas director of manufacturing; more...

The European Fine Chemicals Group (EFCG) and the International Pharmaceutical Excipents Council of Europe (IPEC Europe) announced the formation of a European Pharmaceutical Excipients Certification Project to develop advocacy and stakeholder management in Europe and to give advice to two European working teams as part of an effort to develop a certification program for manufacturers and distributors of pharmaceutical excipients.

Buoyed by increased demand for pharmaceutical outsourcing services, Ricerca Biosciences proceeds with an expansion plan.
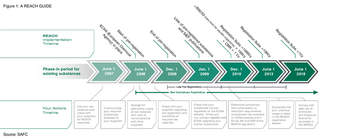
The pharmaceutical industry faces compliance with the REACH regulation, the European Union's regulation on chemicals and their safe use. Fully understanding the requirements, achieving compliance, and developing strategies for working with suppliers are key for avoiding interruption of the supply chain.

Pharmaceutical companies want software vendors to make integrating manufacturing execution systems and enterprise resource planning systems easier.

Industry needs a standard to connect systems and equipment sooner rather than later.
A recent report shows a decline in NASs and a rise in NME applications in 2007.

Planning ahead will ensure successful efforts to improve packaging and packaging operations.

Brief pharmaceutical news items for October 2008.

To err may be human, but to really mess things up, you need management.

Although the European Union's approval rates of biosimilars for market is increasing slower than expected, its approach may provide an example for other foreign markets.
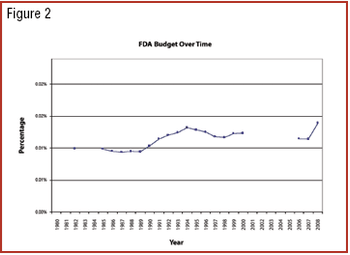
Can previous trends of Democratic and Republican administrations predict industry's future?