
Also, Sartorius Stedim Biotech GmbH to acquire Wave Biotech; AstraZeneca's John Patterson to retire; more...

Also, Sartorius Stedim Biotech GmbH to acquire Wave Biotech; AstraZeneca's John Patterson to retire; more...

Arguments in the Supreme Court case Wyeth v. Levine have concluded, leaving both sides to wait possibly until early 2009 for a decision in a preemption case involving the misadministration of Wyeth's "Phenergan."

The US Food and Drug Administration sent Warning Letters to Bayer HealthCare (Morristown, NJ) about the company's "Bayer Women?s Low Dose Aspirin + Calcium" and "Bayer Aspirin with Heart Advantage" over-the-counter (OTC) products.

The US Food and Drug Administration has issued a final rule requiring the addition of a statement and toll-free number to the labeling of certain human drug products.

Near-stagnant growth for the US pharmaceutical market, the rising influence of emerging markets and specialty products, and increased penetration of generic drugs are major issues for the global pharmaceutical market.

Uncertain economic times crimp the financing flow into the US biotechnology industry.

This position paper describes a model for the future that would provide appropriate standardization, facilitate drug registration and support regulatory agencies.

A well-balanced guide to industrial bioseparations provides valuable information.

Brief pharmaceutical news items for November 2008.

Data capture needs to be fast and reliable...so which automatic identification technology is best?

Attendees at a recent workshop endorsed new methods to detect metals in drugs, dietary supplements, and food ingredients.
With government support, China's pharmaceutical equipment sector is trekking ahead despite challenges regarding the country's overall perceived product quality.

The Indian government may soon monopolize its pharmaceutical industry to cut costs and improve healthcare, but the move is sounding off alarm bells with the companies whose drug products are under review.

The US Food and Drug Administration seeks to understand nanotechnology better and exercise appropriate oversight over products that incorporate it.

Pharma companies could benefit from the lessons learned in this fall's financial crisis.

Production problems come in all shapes, sizes, and ... species.

IPEC Chairman Dave Schoneker discusses current efforts toward facilitating regulatory reviews of new excipients.



Molecules called "chaperones" facilitate correct protein folding.
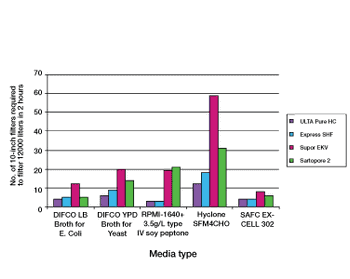
Proper selection of normal flow filters leads to increased process efficiency from early phase product development through to full-scale biopharmaceutical production.

A comparison of conventional cleanrooms, restricted access barrier systems, and isolators, shows the benefits of using isolators in high-potency drug manufacturing.

Scott Sutton discusses the current state of USP ‹1117› and USP's plans for future revisions.

The first 2 weeks of October witnessed the closure of CPhI (30 September–2 October) and the whole of Biotechnica (7–9 October). Pharmaceutical Technology Europe had a presence at both events, which took place in Frankfurt and Hanover (both Germany), respectively. With record-breaking numbers of attendees, roundtables and conferences of the highest quality, and exhibitors from bio and pharma companies from all over the world, these two events are a must in the calendars of many in the biotech and pharmaceutical industries.

Together, Europe and the US account for more than 70% of the global pharmaceutical market, and the growth of these markets is heavily dependent on distribution systems.

The US Government Accountability Office (GAO) released a report last week that examines and provides recommendations for the US Food and Drug Administration's foreign inspection process, including the agency's data management, inspection frequency, and oversight of problems identified during inspections.

The US Food and Drug Administration has awarded the National Institute for Pharmaceutical Technology and Education (NIPTE), a not-for-profit organization comprising 11 universities, a $1.19 million contract to develop quality by design (QbD) guidance elements for design space and scale-up of unit operations.

Merck plans to cut approximately 7200 positions as part of its 2008 restructuring plan, according to an Oct. 22 release focused on the company's third-quarter financial results.

Pfizer and Lilly recently resolved issues related to their marketing practices for certain products.

Also, Maxygen looks to costs, jobs; Receptor BioLogix appoints Dale R. Pfost CEO; more...