
The House Committee on Energy and Commerce issued a revised discussion draft to the Food and Drug Administration Globalization Act of 2008.

The House Committee on Energy and Commerce issued a revised discussion draft to the Food and Drug Administration Globalization Act of 2008.

Next week, the US Food and Drug Administration's communications with pharmaceutical companies will change, and the agency will begin issuing complete response letters in place of approvable letters.

Bristol-Myers Squibb is seeking to acquire the biopharmaceutical company ImClone Systems (New York) for $4.5 billion or $60 per share. BMS currently holds a 17% stake in ImClone.

The US Food and Drug Administration released its user fee rates for fiscal year 2009 last Friday.

Also, Bilcare Global Clinical Supplies opens new headquarters in San Francisco, Sigurdur Oli Olafsson named CEO of Actavis Group, more...

The pharmaceutical industry?s outsourcing practices receive increased attention as Congress evaluates the role of outsourcing in drug-safety issues.

Strong profitability and good growth prospects bode well for the global market for contract research organizations.

Patent pressures, changing disease profiles, and higher costs force companies to fight for the top.

Agents report unusual chemistry, abnormal data analysis, and unconventional work practices.

Myth versus Reality: What does Q10 implementation really mean for my company?

Surrounded by competition, Vietnam's 2020 vision focuses on building a biotech sector worthy of its Asian neighbors-as well as the growing global biopharmaceutical market

Legislative decisions to increase Medicare's formulary may lead to a fight over drug approvals.

USP's guideline for pending monographs can speed up publication of monograhs and time to market.

Despite its flaws, a recent release fills a need for books about pharmaceutical project management.
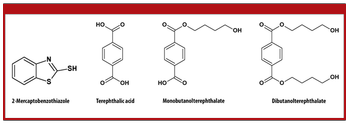
Extractables and leachables are a growing concern for pharmaceutical manufacturers and regulatory bodies.

The European Medicines Agency recommended strengthening the warnings for oral moxifloxacin medicines and concluded that these drugs should only be prescribed to treat acute bacterial sinusitis, acute exacerbation of chronic bronchitis, and community-acquired pneumonia when other antibiotics cannot be used or have failed.

Also, Cobra Biomanufacturing to extend collaboration and form joint venture with ViroMed, Epix Pharmaceuticals CEO resigns, more...

Pharmaceutical companies developing new drug candidates for Hepatitis C virus infection now can test their compounds with a novel culture system that mimics the biology of HCV infection in humans.

As part of a new strategic direction, GlaxoSmithKline CEO Andrew Witty outlined three priorities that are designed to improve the company's long-term financial performance.

Senator Sherrod Brown sent a letter to Richard T. Clark, president and chief executive officer of Merck (Whitehouse Station, NJ), to ask for information about the company's reliance on global outsourcing for the manufacture of pharmaceutical ingredients and finished products.

The US Food and Drug Administration seeks applicants for its new Commissioner’s Fellowship Program for scientists, engineers, and health professionals. The two-year program will help prepare the agency to replace a large number of FDA staff who are preparing to retire as well as meet future goals.

Teva Pharmaceutical Industries and Barr Pharmaceuticals (Montvale, NJ) signed a definitive agreement under which Teva will acquire Barr, the fourth-largest generic-drug company in the world. Teva will buy each share of Barr common stock for $39.90 in cash and 0.6272 Teva shares. Based on the closing price of Teva?s shares on July 16, 2008, the price per share of Barr common stock is $66.50, and the total value of the acquisition is $7.46 billion. Teva will assume Barr?s outstanding net debt of approximately $1.5 billion.

Roche plans to acquire the biopharmaceutical company Genentech (South San Francisco, CA) for $43.7 billion. Roche currently holds a 55.9% stake in Genentech and it plans to acquire the remaining publicly held minority interest that it does not own for $89 per share.

Also, Niro changes its name to GEA Process Engineering; Frances K. Heller joins Exelixis as executive vice-president of business development, more...

The US Food and Drug Administration issued a final rule on July 18 that exempts early Phase I investigational drugs from certain good manufacturing practice regulations.

Pfizer (New York, NY) announced plans to restructure staff at its Kalamazoo, Michigan, facility, leading to an estimated job cut of 275 by the end of the year.

Bristol-Myers Squibb (BMS) agreed to reduce the output of ozone-depleting refrigerants at several industrial facilities around the country to resolve violations of the Clean Air Act. The company's modifications will cost approximately $3.65 million.

3M Drug Delivery Systems has successfully designed a proof of concept device using a solid microstructured transdermal system for the systemic delivery of high-potency pharmaceuticals. The technology was showcased at a poster session at the annual meeting of the Controlled Release Society held this week in New York City.

Also, Roche to end HIV/AIDS research program, WuXi PharmaTech makes appointments, more...

The US Food and Drug Administration published a Draft Guidance for Industry on Providing Regulatory Submissions in Electronic Format in the Federal Register on July 11.