
Also, Alkermes announces restructuring and reduction of workforce, Icagen announces several senior management promotions, more...

Also, Alkermes announces restructuring and reduction of workforce, Icagen announces several senior management promotions, more...

Scientists at the University of North Carolina at Chapel Hill have successfully tested a new inhaled tuberculosis (TB) vaccine.

The US market for prescription pharmaceutical grow only 3.8% in 2007, the lowest growth rate in more than 40 years, according to a recent analysis by IMS Health.

Sandoz introduced its "Omnitrope Pen 5" with liquid cartridge in the United States. The product was approved by the US Food and Drug Administration and is a new form of the first follow-on version of a recombinant biotechnology drug.

The US Pharmacopeial Convention announced a revised glycerin monograph in the United States Pharmacopeia.

As part of ongoing efforts to improve the quality of imported drug products, the US Food and Drug Administration is setting up shop in China.

Also, Pipex Pharmaceuticals implements cost-cutting measures, Pfizer's Senior Vice-President and General Counsel Allen Waxman leaves the company, more...

The US Senate passed an amendment to the fiscal year 2009 budget bill that will increase the amount allotted to FDA by $71 million, bringing the agency's total FY 2009 budget increase to $375 million.

Hoping to provide a better understanding of its role in the heparin contamination investigations, the US Food and Drug Administration has posted a series of information sheets on its website.

This issue of Equipment & Processing Report features products from Eriez Magnetics and Netzsch Fine Particle Technology

When seeking to increase productivity, companies must consider worker–machine and worker–materials interactions. These factors are easy to overlook, but they affect workers' performance and health and a company's bottom line.

Packaging processes, like other pharmaceutical operations, benefit from the speed and repeatability that automation brings. Robotics in particular provide flexibility and accuracy. In some packaging applications such as carton loading, robotics also perform more efficiently than dedicated machines.

This year, INTERPHEX will take place in Philadelphia, Pennsylvania, on March 26?28. Equipment and Processing Report asked RJ Palermo, industry vice-president of Reed Life Sciences and INTERPHEX, to share his thoughts and expectations about the upcoming event.

The US Pharmacopeial Convention (USP) signed a Memorandum of Understanding (MOU) with the Chinese Pharmacopoeia Commission (ChP) designed to strengthen the quality of medicine and foods in the United States and China.

The US House of Representatives Committee on Homeland Security approved legislation last week that mandates inherently safer technologies (IST) as part of chemical-site security standards, a move that was opposed by the Synthetic Organic Chemical Manufacturers Association.

Concerns over contaminated heparin product worsened as US officials announced dialysis patients in Germany have fallen ill after using a different brand of the blood thinner than was already being recalled.

Also, PDL BioPharma will no longer pursue sale of the company, executives resign from Topigen Pharmaceuticals, more...

Much to the surprise of most close observers of the Food and Drug Administration, Janet Woodcock agreed last month to resume control of the Center for Drug Evaluation and Research.

Eli Lilly terminated the development of its inhaled insulin product AIR, a diabetes treatment that had been in Phase III clinical trials.

The 59th Pittsburgh Conference gathered more than 1000 exhibitors on its showroom floor this week.

Also, Millipore plans to open Singapore facility, Michael J. Simms joins Alexza Pharmaceuticals, more...

Merck & Co. advised AstraZeneca (London) that it will not exercise its option to sell its interest in certain AstraZeneca non-proton pump inhibitor (non-PPI) products this year.

With another potential "made-in-China" crisis looming over the recall of heparin, critics and the media seem to be waiting in line to take another jab at the US Food and Drug Administration.

Leveraging its global infrastructure, SAFC Supply Solution pilots a new external sourcing program.

Draft federal legislation that would require high-risk chemical facilities to use inherently safer technology for reducing their risk may present potential problems for custom and batch manufacturers supplying the pharmaceutical industry.

After two centuries, there's no reason to maintain two tablet compression tooling standards.

A. Nair discusses patent disputes in India.

If not properly monitored, filters and plastic bags can keep back more than they should.

The United States Pharmacopeia emphasizes mechanical calibration and a performance test to esnure integrity of the dissolution procedure.
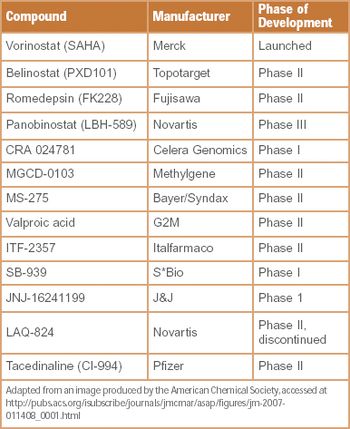
New research and ideas for March 2008