
Washington, DC (Sept. 1) - In a Federal Register announcement the US Food and Drug Administration laid out its guidance agenda for the coming months.

Washington, DC (Sept. 1) - In a Federal Register announcement the US Food and Drug Administration laid out its guidance agenda for the coming months.

Arthur R. Mlodozeniec, PhD, a member of Pharmaceutical Technology's advisory board and past president of AAPS, died on Sept. 4.

The overall market size of the Southeast Asia region totals $7 billion, with a projected compound annual growth rate of about 13% through 2010.

Multinational, brand-name drug companies dominate 70% of the whole market, whereas local and generic drug companies dominate 30% of the market.

China's new Regulation for the Administration of Drug Packaging includes significant changes from the previous rule.

What if your country, even amid political turmoil, has been growing at a fast pace over the last several years and keeps doing quite well, but is nevertheless home to more than 14 million people living below the poverty line? What if, given this context, the retail prices of medicines are among the highest in the world and the major distributor is granted the privilege of a near monopoly? And what if, because of a huge predominance of multinational companies, lower-priced generic medicines are struggling to establish themselves (even though you passed a "generic drugs law" back in the '80s)? Well, if you are a proud and committed citizen of such a country and, above all, you deal on a daily basis with pharmaceutical-related issues, you must be very disappointed, if not upset. And yes, it's quite probable that you are a Filipino, as Roberto Pagdanganan, chairman of the Philippine International Trading Corporation (PITC), is. In fact, for a foreign observer, the problem of drug prices is one of the most..

Mid-size companies are forcing through their market positions by developing their niche competencies and their flexibilities to be recognized as serious market contenders.

Select large custom manufacturers expand capacity, private equity firms buy companies in transition, and players from India and China build their positions.

A major contributor to his most recent election victory, Thai Prime Minister Thaksin Shinawatra's "30 Baht Health Plan," first announced in 2001, was said to be born more out of populist politics than actual necessity for healthcare reform. This health plan is now draining the state-run hospitals, plunging them into perpetual debt and creating a mass exodus of overworked, underpaid doctors to the private sector. Nonetheless, this unprecedented universal healthcare policy's saving grace has been to provide affordable healthcare to nearly 50 million Thai, according to 2005 estimates by the National Health Security Office (NHSO).

I recently embarked on a quest: to investigate industry's use of the words, "generic" and "biosimilar" when describing a biologic molecule. An English major at heart, I was wrapped up in a news story that was partly about science, partly about words.
FDA will strengthen its collaborative relationships with federal agencies participating in the National Nanotechnology Initiative.

No matter how much we scamper about, the sheep just won't go where we want them to.

Apparently, the inspector would sneak off to visit his relatives on FDA time, instead of visiting us.

Now is the time to get ahead of the learning curve and explore options for meeting pedigree guidelines.
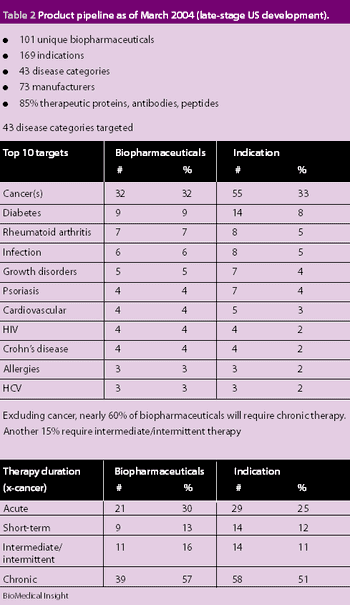
The worldwide market for biopharmaceuticals was estimated to be $50 billion in 2005. North America accounts for 60% in terms of revenue and R&D. Europe accounts for 20% and Japan 10%. It is also estimated that 400–500 biotech drugs are under clinical development for various disease conditions. Biopharmaceuticals are being developed to fight cancer, viral infections, diabetes, hepatitis and multiple sclerosis. The distinct families of biopharmaceuticals include

It's been a while since I last wrote my last editor's comment - a combination, during the last few months, of attending a variety of industry events (as the conference and exhibition season peaked before the summer) and being on holiday. I apologize for the poor excuses, but it's certainly better than saying the dog ate my homework.

With a finger on the pulse of an evolving market, the sales and marketing team can visually demonstrate trends and new market opportunities to the R&D team

Cardinal Health halted production, sales, repairs, and installations of its "Alaris Signature Edition Gold" infusion pump after the US Food and Drug Administration (Rockville, MD) seized approximately 1300 units last Friday. The seized infusion pumps (model numbers 7130, 7131, 7230, and 7231) have a "key bounce" defect that may cause overinfusion of medications by more than 10 times the intended infusion rate.

The generic drug company Mylan Laboratories, Inc. (Pittsburgh, PA) plans to acquire a controlling interest (71.5%) in the Indian pharmaceutical company Matrix Laboratories, Ltd (Hyderabad, India) for $736 million.

Hemispherx Biopharma, Inc., Boehringer Ingelheim Chemicals, Inc., MedImmune, Inc.

Biovail Corporation is demanding that the US Food and Drug Administration (Rockville, MD) enforce the proper criteria for determining bioequivalence of extended-release generic versions of bupropion products.

Merck KGaA (Darmstadt, Germany) plans to invest 190 million euros ($245 million) to build a biopharmaceutical plant in Darmstadt, Germany. Production is expected to begin in 2010.

Washington, DC (Aug. 14)?A new report issued by the Pharmaceutical Research and Manufacturers of America identifies 418 drugs and vaccines developed through biotechnology. All of the biotechnology medicines and vaccines are now in clinical trials or awaiting approval by the US Food and Drug Administration (Rockville, MD).

West Point, PA (Jul. 31)?A survey of 150 US pharmacists found that nearly 80% believe that "multiple look-alike medications" make it difficult for consumers to identify the correct medication, especially when the drugs are moved to unlabeled containers.

Teva Pharmaceutical Industries Ltd. announced that the company will cease production at its manufacturing facility in Cidra, Puerto Rico during the fourth quarter of 2006.

The International Organization for Standardization (ISO, www.iso.org) has formally issued and published standard ISO 4644-8:2006, Cleanrooms and associated controlled environments-Part 8: Classification of airborne molecular contamination. The document covers the classification of airborne molecular contamination (AMC) in cleanrooms and associated controlled environments in terms of airborne concentrations of specific chemical substances (individual, group, or category) and provides a protocol to include test methods, analysis, and time-weighted factors within the specification for classification.

Air Products, Althea Technologies, Inc., Bradley Pharmaceuticals, Bristol-Myers Squibb, Crucell N.V., Emergent Biosolutions

IBM Corporation (www.ibm.com) has combined radio-frequency identification (RFID) software, technology, and services to develop a track-and-trace system for pharmaceutical products.

Auriga, Balchem, Generex, ImClone, Intranasal Therapeutics, MedImmune, Schering

The US Food and Drug Administration's New Jersey District Office issued a Warning Letter to Concord Laboratories (Fairfield, NJ), citing the company for manufacturing three generic products without an ANDA, and for ten deviations from current good manufacturing practices.