
Because disposable systems offer benefits that can impact the time for development as well as for the cost of production, the biotech sector is expected to continue to use these technologies ...

Because disposable systems offer benefits that can impact the time for development as well as for the cost of production, the biotech sector is expected to continue to use these technologies ...

Two of the largest companies in the pharma industry are to join forces to supply and distribute 96-well Multi-SPE Extraction Plates. The instruments, which are to be circulated by Millipore, are manufactured by 3M for the purpose of isolating and concentrating low levels of analytes, and function by removing substances that interfere with mass spectrometry signal detection. The devices feature 3M Empore membranes, which produce clean extracts, extend LC-MS/MS column life and decrease instrument downtime.

Chiron Corporation (Emeryville, CA, www.chiron.com) announced that quality problems at its Marlburg, Germany, manufacturing plant will prevent the company from supplying its "Begrivac" influenza virus vaccine to non-US markets for the 2005–2006 flu season.

As one Midwestern city has discovered, there is life after Big Pharma.

Our GMP Agent-in-Place at a top-10 pharmaceutical manufacturing firm reports on a spill during the manufacture of a time-release capsule filled with coated beads.

Cold chain management is becoming pivotal to success in the pharmaceutical industry.

To help meet the needs of the fast-growing, global biopharmaceuticals industry, Pall Corporation has launched five innovative technologies aimed at increasing drug-manufacturing efficiency through customization and disposability. The Mustang XT5000 capsule is the company's novel chromatography technology for efficient capture of large molecules, especially used to develop DNA and virus-based drugs. Expanding its portfolio of technologies for disposable processing, Pall offers both the customizable Tangential Flow Filtration system, which incorporates single-use components for downstream processing applications, and the Kleenpak Connector — a new single-use device, which enables aseptic connections to be made instantly.

API and fine chemicals companies in Asia are flexing their muscles in a bid to increase their market share in key territories. This has been demonstrated by India's Malladi Drugs and Pharmaceuticals, which earlier this summer acquired Novus, a US company, to boost its presence in the pseudoephedrine (an ingredient used in cough and cold formulations) market. Reportedly, this is the first time (and it certainly won't be the last) that an Indian API manufacturer has bought a US counterpart and drives home the message that Indian companies are looking at providing products on a global scale. The deal gives Malladi an almost vice-like grip on a third of the global pseudoephedrine market, and more importantly, a foot in the lucrative US sector.

Colorcon has opened three specially equipped design centres devoted to tablet brand imaging. Located in West Point (PA, USA), Dartford (UK) and Goa (India), the facilities form an integral part of the brand enhancement system for tablets — a unique service supporting new product development and brand management of an existing product. Each design facility is equipped for collaboration between Colorcon experts and a client's own project team.

More and more often, manufacturers in need of outside services are turning to preferred providers.

Able Labs Files for Chapter 11

Xanodyne Acquires aaiPharma Assets


Able Interim Chief Resigns Amid Drug Recall

Police and protests at BIO 2005.

The industry should develop a set of best practices for managing information technology systems and not wait for FDA to take the lead.

Able Laboratories, Inc. (Cranbury, NJ, www.ablelabs.com) announced in late May that it would halt all production and recall all available product because of problematic testing procedures discovered during an internal investigation. The company manufactures mostly generic prescription drugs, including drugs containing acetaminophen.

Schering AG To Cut Plants and Staff

Wyeth and Purdue Announce Restructuring Plans

Pharmaceutical science and technology news.

Regulation: FDA Steps Up Action Against Counterfeit Drugs, Warns US Consumers Against Fake Medicine from Mexico

A myopic focus on short-term growth can endanger the community. The question is, can we enlighten our self-interest before the edifice falls down around our ears?

Once considered mainly an afterthought in a company's lifecycle-management strategy, controlled-release dosage forms are now positioned at the forefront of many formulation strategies. In contrast to drug discovery, formulation work focuses not only on the intricacies of the active pharmaceutical ingredient (API), but also on fine-tuning the excipients, the release profile, and the delivery mechanism to provide optimal therapeutic benefit. Because of their wide range of applications and functionalities, especially in controlled-release therapies, polymers are among the most widely used excipients.

In his Feb. 2005 viewpoint article, "In Defense of Singlet Testing," Torbeck (1) draws an important philosophical distinction between "standards" and "specifications." He argues that specifications are criteria selected by manufacturers for statistical control of their products, whereas compendial standards are absolute requirements. This distinction is entirely compatible with modern concepts of statistical process control.

Purdue?s RFID Pedigree Program Enters Pilot Phase

Detection and identification of different polymorphic forms is, therefore, important throughout the drug development and manufacturing process.
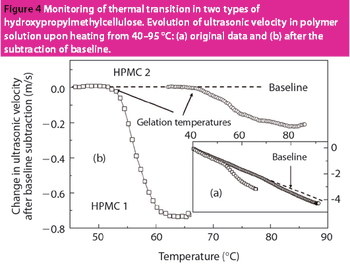
HR-US is a nondestructive technique with enormous potential for the analysis of materials and formulations used in the pharmaceutical industry.

Possible cross-contamination issues should be eliminated at the early stage of the project. The project sponsor should ensure that all relevant personnel from the production, quality control, logistics, and maintenance departments, as well as engineering, are involved in the conceptual stages of a design.
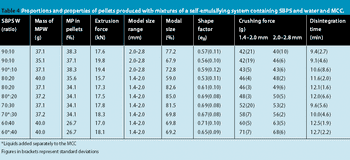
This high quality of pellet roundness is surprising in view of the high extrusion forces required to extrude the wet masses.