
The authors developed a metronidazole-based floating drug-delivery system to investigate the effect of rate-controlling polymers on release pattern and duration of buoyancy in matrix tablets.

The authors developed a metronidazole-based floating drug-delivery system to investigate the effect of rate-controlling polymers on release pattern and duration of buoyancy in matrix tablets.
The study evaluates near-infrared analysis of tablets nominally containing 4 mg of chlorpheniramine maleate and 10 mg of phenylephrine hydrochloride per dose.
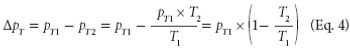
The authors describe the operational qualification of test accuracy with regard to temperature drift using a thermal-compensation algorithm on several freeze dryers.
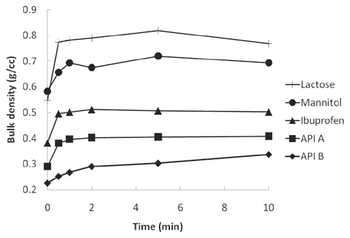
The authors experiment with a resonant acoustic mixer as a method for dry powder coating.

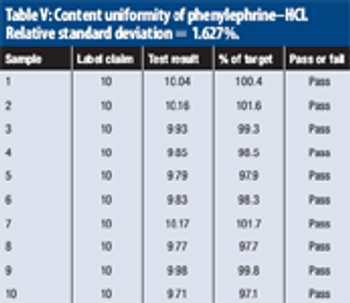
The authors describe the concept of the limiting discriminatory the limiting discriminatory threshold (LDT) as an objective means of evaluating the inherent quality requirement of a large-sample content-uniformity test.
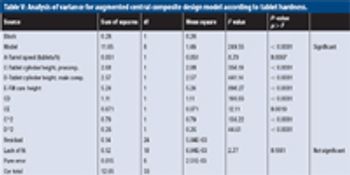
The author prepared and analyzed a detailed design of experiments for the manufacture of a simple tablet formulation. The aim was to test whether tablet hardness and weight could be controlled during the compression process by adjusting certain machine parameters.
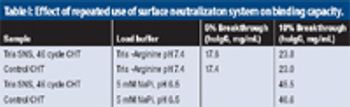
A new, robust method for protein elution from ceramic hydroxyapatite.
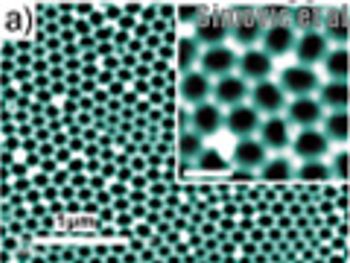
The authors present a method for controlling the release of therapeutics by applying a plasma polymer layer to the surface of porous materials.

This study demonstrates the beneficial use of a spatial-filter velocimetry particle-size analyzer during granulation.
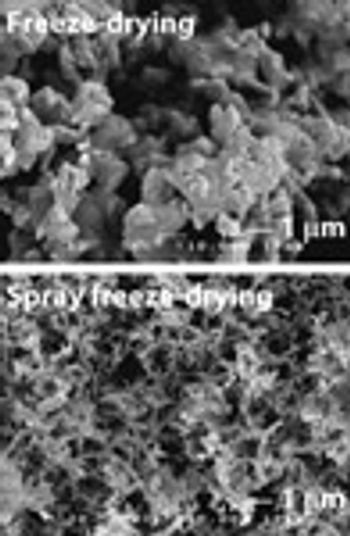
The authors discuss the preparation of lipophilic drug nanocrystals by controlled crystallization during freeze-drying.

The authors investigate the influence of hydro-alcoholic media on hydration and drug release from polyethylene oxide extended-release matrices.
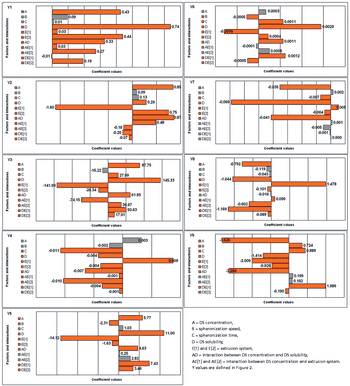
The authors compare three systems of single-screw extrusion using binary formulations for their suitability for producing pellets of various formulations and under various spheronization conditions.
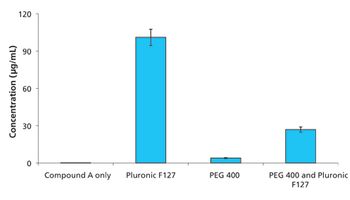
The study results suggested that Pluronic F127 might be a potent inhibitor of drug precipitation for Labrasol formulations.
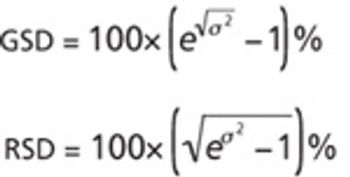
The author argues that traditional concerns about repeatability and intermediate precision remain valid but insufficient.
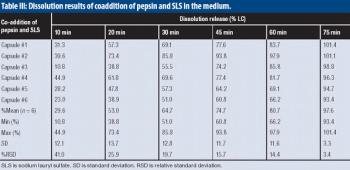
The authors develop a pratical approach to avoid unwanted interactions between pepsin and SLS in dissolution Tier II tests.
The authors examine the influence of glass-transition temperature, melt viscosity, degradation temperature, and process settings.
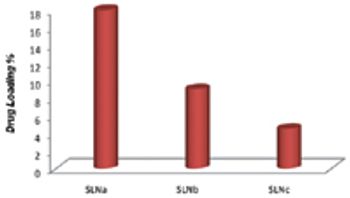
The aim of this study was to prepare and characterize physiochemically and biologically tamoxifen-loaded SLNs to evaluate their effectiveness as a drug-delivery system to treat breast cancers.

The author presents a method to calculate the relationship between supply air volume flow and airborne particle concentrations.

Although tablet manufacture is traditionally a batchbased wet granulation process, there are many advantages to be gained by adopting dry granulation, including lower costs and increased yields.
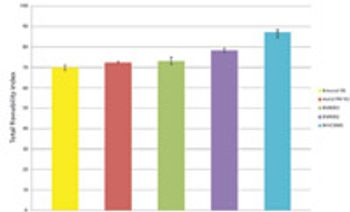
The authors investigated the tableting properties of PanExcea MHC300G, a high-performance excipient.

The author challenges current detection methodologies.

The way in which a suspension nasal spray product interacts with the body depends not only on the droplet size of the delivered droplet, but also on the particle size of the suspended API.
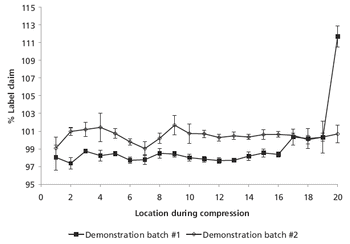
The authors modified equipment and the manufacturing process to re-establish content uniformity among tablets.

The authors describe a new method for the production of personalised medicines utilising ultra-precise microdrop printing technology.

The authors discuss how their research will help FDA in its identification of areas of emphasis in pre- and postapproval evaluation of products and processes.

The authors conducted an experiment to determine the type of extrusion that provides the best productivity and pellet quality. This article contains online bonus material.
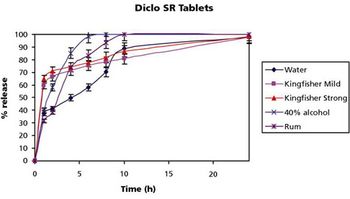
The authors evaluated the effect of alcoholic beverages on the release profiles of sustained-release dosage forms containing metformin and diclofenac.

With the increasing need for businesses to reduce costs and demonstrate value, there is a requirement to look at all aspects of bio/pharma drug development and manufacturing to achieve efficiency improvements.
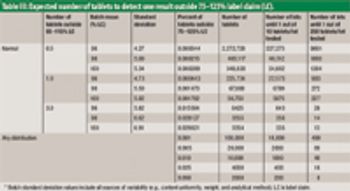
The authors describe a modified version of the Large-N test used to determine content uniformity.
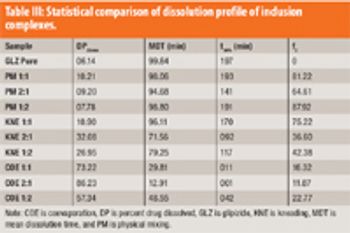
The authors describe the development of an inclusion complex of GLZ and formulated an extended-release dosage form based on osmotic technology.