
The authors developed a reliable spectrophotometric method for determining and measuring trace amounts of lead in various samples.

The authors developed a reliable spectrophotometric method for determining and measuring trace amounts of lead in various samples.

Regulatory agencies expect companies to establish and monitor clean equipment- and dirty equipment-hold times for manufacturing equipment as part of their cleaning validation program.

There are significant differences between the United States and European Union requirements for the annual review of records related to the manufacturing and control for pharmaceutical products and active pharmaceutical ingredients.
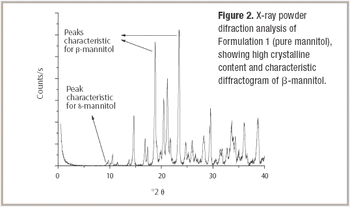
Mannitol is the most commonly used bulking agent in freeze-drying formulation design. The benefit of using mannitol is that it crystallizes during freezing and permits drying processes at higher product temperatures, and thus with higher sublimation rates relative to purely amorphous systems (1). Mannitol, however, is known to form different crystalline modifications which compromises reproducibility of product characteristics and storage stability due to phase transformations (2, 3).
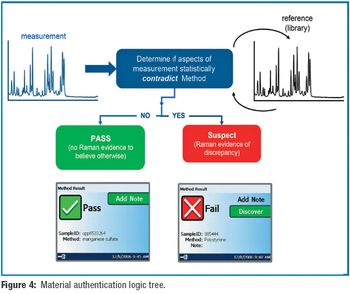
Using a handheld Raman spectrometer, the authors developed methods for 28 commonly used excipients and active ingredients.

The selection of an appropriate salt form for a potential drug candidate is an opportunity to modulate its characteristics to improve bioavailability, stability, manufacturability, and patient compliance.

In this topical review, the authors discuss the rationale behind microstructural requirements for biopharmaceutical equipment and problems that may be encountered during the fabrication of high-performance corrosion-resistant equipment.
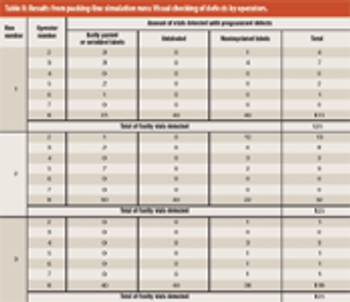
This article focuses on upgrading and improving a packing process to comply with current good manufacturing practices. The authors sought to maintain proper quality assurance for finished products.
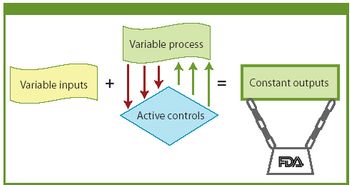
FDA is modernizing and streamlining current good manufacturing practices. The author examines FDA's evolving approach to quality systems and how a manufacturer can implement a quality system framework.
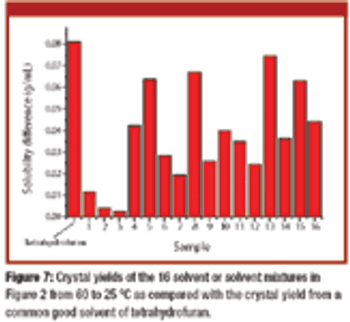
The authors propose extending initial solvent screening for a single-solvent system to the cocktail solvent screening of binary and ternary solvent mixtures.

Natural gums and mucilage are biocompatible, cheap, readily available, and represent a potential source of excipients. The authors examine the functionality of mucilage extracted from the leaves of Hibiscus rosa-sinensis Linn as an excipient in a sustained-release tablet formulation.
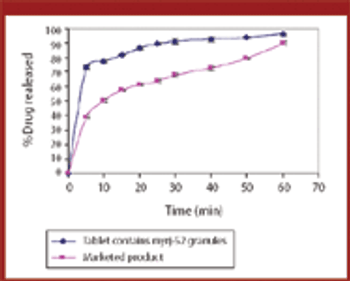
Various manufacturing techniques can improve a drug's solubility, thus increasing its bioavailability. The authors examined whether melt granulation can enhance drug solubility using meloxicam as the drug substance and myrj-52 as the binder.
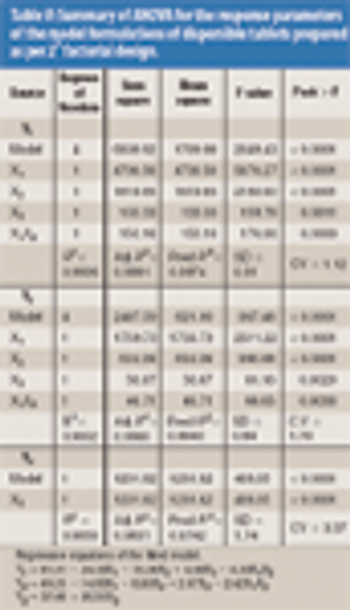
The authors analyzed the effects of complexation as well as the levels of ammonium bicarbonate and crospovidone on tablet wetting time (WT), disintegration time (DT), and percent dissolution efficiency at 60 min (%DE60).

To monitor and control processes or products, analytical methodology must be fit for purpose. An approach to apply quality by design principles to the design and evaluation of analytical methods has therefore been developed to meet these needs. This article features a downloadable template on which to conduct a failure mode effect analysis (FMEA).
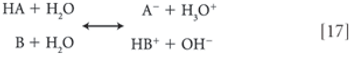
Through consideration of the ionic equilibria of acids and bases, one may readily calculate the formation constant of a salt species solely on the basis of knowledge of the pKA value of the acid and the pKB value of the base.