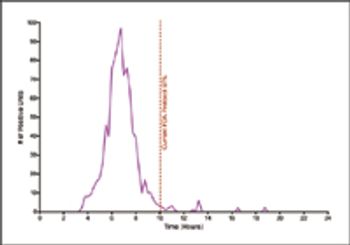
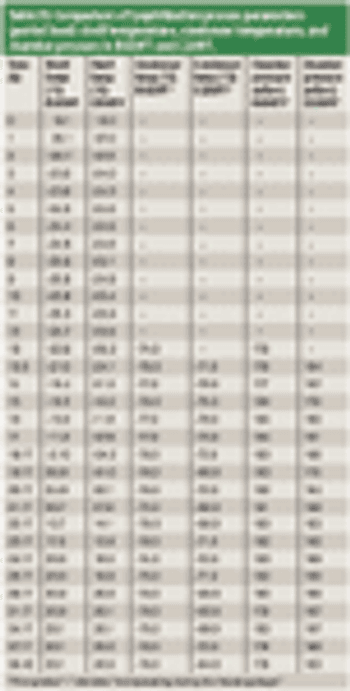
This study used biological indicators containing Geobacillus stearothermophilus spores and a new technology to continuously monitor incubated BIs and record nonsterile results.

This study used biological indicators containing Geobacillus stearothermophilus spores and a new technology to continuously monitor incubated BIs and record nonsterile results.
In this article, the authors evaluated the effects of the granulating binder level, binder type, water amount, and water-droplet size on the MADG process.

The authors discuss a study that demonstrates the use of polyethylene oxide mixtures to investigate the effect of polymer viscosity on formulation robustness.

The quality assurance environment is forcing pharmaceutical companies to face new challenges. In light of this, the authors conducted a study of professionals from some of the world's top pharmaceutical companies to identify key QA concerns.

Manual sample preparation methods for solid dose pharmaceuticals have a number of inherent disadvantages, primarily because they are time consuming and unpredictable.

The application of roll compaction as a dry granulation method for three different drug types — herbal dry extract, poorly compactable drug and a sustained-release matrix tablet &#amp;151; was examined.

The authors developed a robust, automated system to conduct Karl Fischer moisture assays for lyophilized products.
The authors explain a process for moisture-activated dry granulation in detail and provide guidance for the selection of excipients and equipment.

Chronotherapeutic drug delivery systems (CRDDS) have been recognized as potentially beneficial to the chronotherapy (timeoptimized therapy) of widespread chronic diseases that display time-dependent symptoms.
The authors investigated the effects of formulation and processing parameters on floating matrix-controlled drug-delivery systems.

Using handheld Raman spectroscopy, methods were developed and evaluated for 198 substances widely used as raw materials.

Applying a moisture protective barrier to tablet cores can help prevent degradation caused by ambient moisture.

Endotoxin removal from a finished product is a major challenge for biopharmaceutical manufacturers; particularly as all endotoxin removal methods have operational limitations and may result in loss of protein.

Formoterol presents formulators and manufacturers in the asthma and chronic obstructive pulmonary disease marketplace many challenges.
The authors describe a comprehensive methodology for establishing functional equivalence among various lyophilizers.

Parteck ODT is a newly introduced ready-to-use excipient for fast melt tablets.
Human embryonic stem cells are of immense interest to researchers because of their ability to potentially develop into any kind of tissue.

When validating automated systems from third-party providers, using the V model and failure modes effects and criticality analysis (FMECA) early in the process can help.

The addition of superdisintegrants to oral solid dosage forms can improve disintegration and, in turn, drug dissolution.
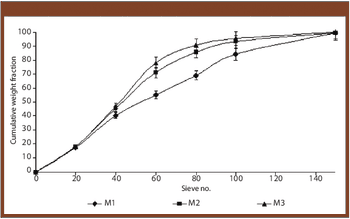
The authors prepared granules containing cinnarizine using polyethylene glycol 6000 as a melting binder and lactose monohydrate as hydrophilic filler. The effects of binder concentration and size were studied.
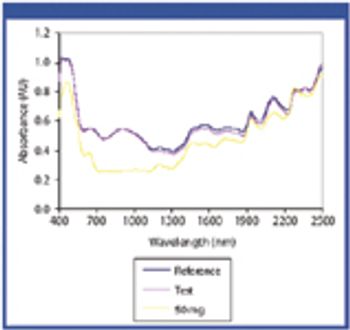
The authors applied near-infrared (NIR) spectrophotometry to assess whether eight drug products were authentic or counterfeit.

This article provides an overview of the drug Recall Root Cause Research (RRCR) project, an initiative of the US Food and Drug Administration's Center for Drug Evaluation and Research.
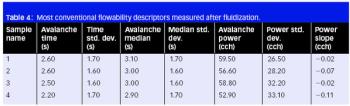
The authors modified gellan gum using microwave technology and showed it can be used as an excipient in tablet formulations.
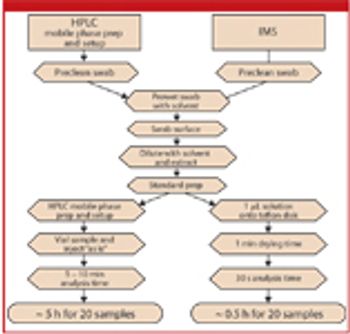
The authors discuss the theory of ion mobility spectrometry, its benefit over HPLC analysis in cleaning verification, and the experimental considerations for method validation and validation.
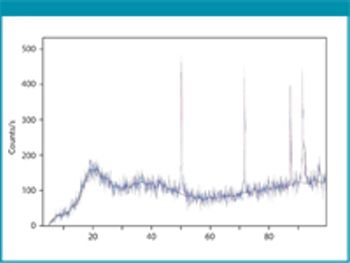
Cefaclor is a β-lactam cephalosporin antibiotic that has a wide particle size distribution. Because of the nonporous nature of the material, the specific surface area value accounts for a significant amount of fine particles possibly present in the samples under analysis.
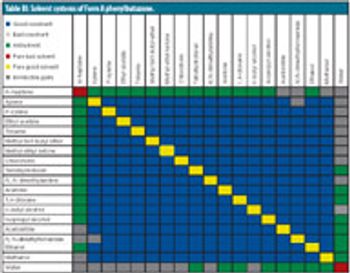
The authors describe the importance of a rapid and an abbreviated screening strategy in initial solvent screening. This article contains bonus online-exclusive material.
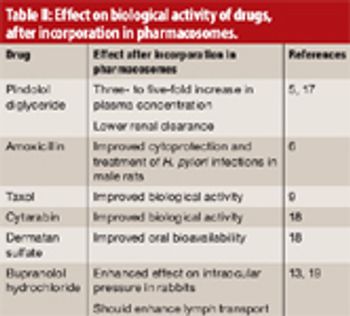
Pharmacosomes can pass through biomembranes efficiently and possess several advantages over traditional vesicular drug-delivery systems.
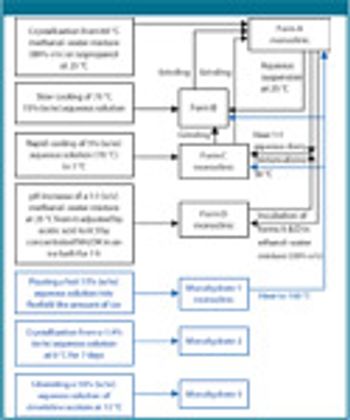
The authors describe the importance of a rapid and an abbreviated screening strategy by initial solvent screening in 20-mL scintillation vials.
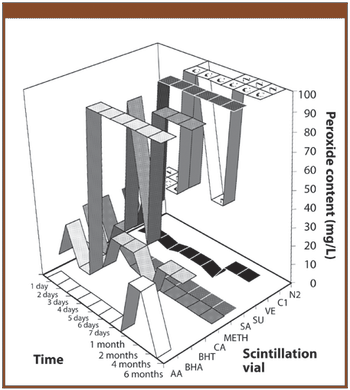
The authors investigate whether the addition of an antioxidant could be used to stabilize the solvent ethyl lactate by preventing the formation of peroxides
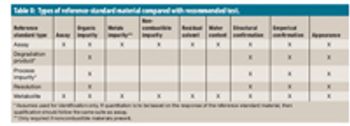
The author reviews the types of reference-standard materials used in drug-product manufacturing, discusses current regulatory requirements, and outlines a reference-standard qualification program.