
The authors demonstrated that ODTs can be obtained by direct compression of a mixture of starch, fructose, and SMCC.

The authors demonstrated that ODTs can be obtained by direct compression of a mixture of starch, fructose, and SMCC.

Although there are no regulatory requirements or established pharmacopoeial techniques for the dissolution testing of inhaled drugs, such testing can potentially open up the opportunity to tailor formulation properties.

This article examines the difficulties in designing lyophilisation processes that can be faithfully scaled up to production volumes and suggests the most effective ways in which this can be achieved.
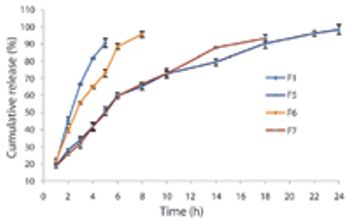
The authors developed a formulation for effervescent gastroretentive drug delivery techniques using ibuprofen as a model drug. They optimized the formulations by applying full factorial design.
There are apparent inconsistencies between the use of these reference materials and the manufacture of the reference solutions that are actually used in the regulated test method.

Using new co-processing technologies, the authors show that it is possible to formulate an 'all-round' excipient.
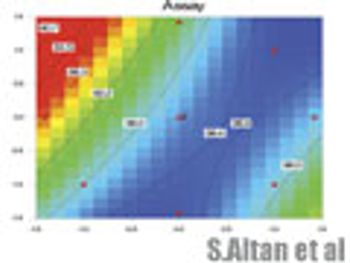
In the final article of a three-part series, the authors discuss how to present a design space and evaluate its graphical representation.

Utilizing integrated active radio frequency identification (RFID) and real-time asset management systems can yield commercial and compliance benefits.

A case study describing how Lean can drive the creation of an improved culture within pharmaceutical companies.

One particularly crucial parameter for nasal sprays is the size of the droplets produced during actuation, which can potentially impact bioavailability.

The authors discuss the statistical tools used in experimental planning and stategy and how to evaluate the resulting design space and its graphical representation.

The authors used common solvents to develop an initial solvent-screening method for laboratory-scale research to determine the solubility, polymorphism, and crystal properties of various active ingredients.
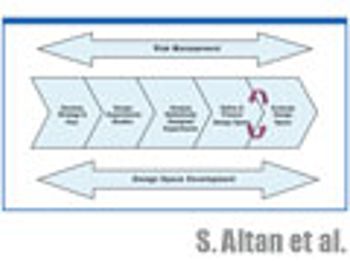
The authors discuss the statistical tools used in experimental planning and strategy and how to evaluate the resulting design space and its graphical representation.

The functionality and performance of three types of commercial superdisintegrants were evaluated in the application of orally disintegrating tablets.

Technical Note: The authors investigated the variables important for calcium-alginate formation as well as dissolution.

How can crystal engineering and pharma sciences be combined to enhance the clinical performance of drugs?
The authors recommend a strategy for classifying similar nonstainless-steel surfaces into three groups based upon the analytical recovery that was observed in this study.

An effective quality management system will identify the root cause of non-conformances and put measures in place to ensure that they do not recur, but is the pharmaceutical industry choosing the right methods to make sure this happens?

An environmental risk assessment (ERA) is now required for all new pharmaceutical product marketing authorisation applications. The author outlines the steps to this procedure.
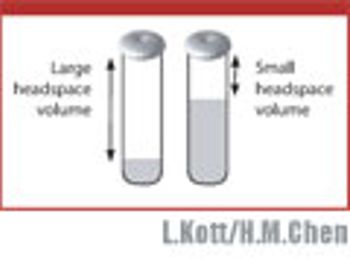
In this case study of amines, the authors discuss several parameters to be considered in developing a headspace GC method.

The authors demonstrate that sustained-release delivery can help avoid the risk of sudden higher-blood concentration of a drug to avoid toxicity.

Testing the dissolution rates of a pharmaceutical formulation (also known as in vitro availability) aids drug quality control and is a compulsory requirement of the British, European and US pharmacopoeias.

Though the pharma industry has improved its change management processes, there are still opportunities for improvement.

In Part I of this article, which appeard in the March 2010 issue, the authors describe their approach for constructing form spaces for carbamazepine, cimetidine, and phenylbutazone by initial solvent screening to evaluate the feasibility of spherical crystallization. Part II of this article discusses their findings.
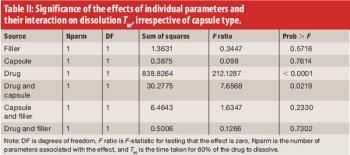
The authors examined the disintegration and dissolution profiles of propranolol and rofecoxib tablets overencapsulated with standard hard-gelatin capsules and with capsules specifically designed for double-blind clinical trials.
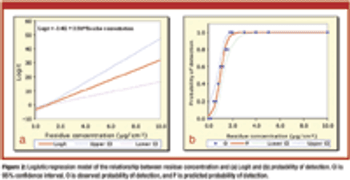
Current methods for establishing visible residue limits (VRLs) are not statistically justifiable. The author proposes a method for estimating VRLs based on logistic regression.
In Part I of this article, the authors describe the materials and methods used in developing a screening strategy to accelerate the preparation and characterization of spherical agglomerates by spherical crystallization.

A new kind of gastro-retentive dosage form for drugs with poor aqueous solubility was developed and evaluated, with the aim of achieving gastro-retention.
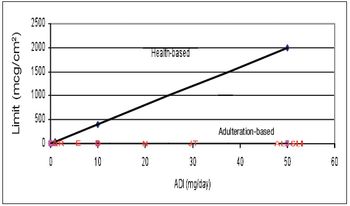
The author discusses how the use of a visible residue limit has made the 10-ppm cleaning limit obsolete in many applications.

Statistics are often viewed as confusing and complicated, but multivariate data analysis (MVA) methods can be used to amass knowledge simply.