
Bio-Rad’s EconoFit Chromatography Column Packs support resin screening experiments in the development of protein purification workflows.

Bio-Rad’s EconoFit Chromatography Column Packs support resin screening experiments in the development of protein purification workflows.

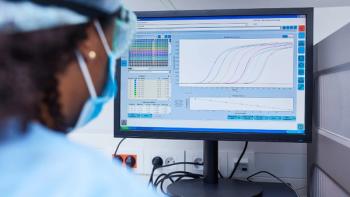
Integrating digital technologies into lab environments can ease workflow and enhance data capture for researchers.

Biosimilar data analysis must demonstrate a correlation between structure and function.

To ensure patient safety, drug products must be tested for elemental impurities that pose risk.

Single-cell analytical technologies can deepen the understanding of cell biology and, therefore, disease mechanisms.

By understanding potential material change, the impact on patient safety can be understood and mitigated.

Systemic inefficiencies in the pharmaceutical industry undermine problem-solving efforts.

Inceptor Bio and the University of Minnesota aim to build a novel iPSC platform to accelerate cell therapy drug development.

Aizon Digitize is designed to help life science manufacturers transition data from paper to digital.

Avid Bioservices has expanded its viral vector development and manufacturing facility with the addition of analytical and process development suites.

Bio-Rad’s SEQuoia Express Stranded RNA Library Prep Kit is designed to construct efficient RNA sequencing workflows.

Axol Bioscience’s CiPA-validated ventricular cardiomyocytes can be used as a robust cardiotoxicity measurement tool.
This article describes the in-vitro permeation test study data processing procedures and FDA statistical mathematics of evaluating a generic topical drug product, acyclovir cream, against its reference product.

Analyzing elemental impurities in drug products is—much like other analytical testing—primarily aimed at ensuring patient safety.
When considering whether to outsource work to a CRO, there are a number of factors to assess, including the type of work that may be outsourced, regulatory considerations, and needs for the study.

BioRad Laboratories CFX Duet System is designed to help researchers develop singleplex and duplex quantitative polymerase chain reaction assays.

Dissolution testing has seen recent trends that have led to increased data integrity solutions as well as an increase in biorelevant testing techniques.

FDA continues efforts to incentivize drug manufacturers to follow higher data integrity requirements.

Through the Thermo Fisher Scientific and LabShares Newton partnership, life science startups can accelerate early-stage discovery and development with shared lab spaces.

PerkinElmer has expanded its genomics testing services with the launch of its ultrarapid whole genome sequencing.

Eurofins partners with PAMM to strengthen laboratory services for the Dutch healthcare system.

Sferalp will use Fluid Air’s PolarDry technology for powder processing.

908 Devices and CPI have formed a collaboration to optimize cell culture media to allow for improved process control.
In this episode of Drug Digest, Pharmaceutical Technology editors, Felicity Thomas and Feliza Mirasol, examine the topic of emerging therapies in more detail, covering subjects such as the challenges of scale, the potential benefits of drug delivery innovation, importance of early analytical studies, the evolution of the regulatory landscape, and differences between regional regulatory requirements.

The “cloud lab” is a virtual laboratory setting that can enable scientists to more quickly advance research.