
Dynamic testing and advances in shear testing provide better insight into powder physical properties and external variables that affect powder behavior.

Dynamic testing and advances in shear testing provide better insight into powder physical properties and external variables that affect powder behavior.

Access to extremely low and consistently controlled reaction temperatures can improve product yields and selectivities while also reducing costs.

Applications of ZFN technology in biopharmaceutical cell-line engineering.

The rejection by India's Supreme Court on Novartis' Glivec/Gleevec (imatinib mesylate) and other recent case law raise important issues on patent strategies for solid forms.

Because peptoids are relatively easy to synthesize, have tremendous sequence diversity, and are more proteolytically stable than peptides and proteins, they have significant potential for use as APIs, drug-delivery agents, and in numerous biomedical applications.

Shimadzu's LCMS-8040 combines newly improved ion optics and collision cell technology with proprietary ultrafast technologies.

Access to simpler and more direct methodologies for the incorporation of fluorinated substituents remains an unmet need in pharmaceutical synthesis. An analysis of recent trends in fluorination chemistry.
Continuous flow chemistry offers potential for greater control, improved safety and environmental profiles, and efficient chemical transformations.

Contract API manufacturers proceed with select investment in capacity and service additions.
This article summarizes the development and modernization of the United States Pharmacopeia-National Formulary (USP-NF) fixed-oil excipient monographs. This article contains bonus online-exclusive material.

A Q&A with Tony Hitchcock, head of manufacturing at Cobra Biologics.

The 2013 Excipient Information Package (EIP) User Guide is now available for free download from the International Pharmaceutical Excipients Council (IPEC)-Americas.

Roche and BioLamina have entered into a research and development agreement to jointly develop new cell culture systems for various applications, including stem cell research. The collaboration will assess laminin-based in-vitro cell culture matrices that can offer physiological microenvironments for living cells.

Visual mapping can provide a particle-size distribution estimate.

Growth is seen in outsourcing of insect- and plant-cell-based bioproduction expression systems.

Factors for assessing excipient variability, the associated challenges developers need to address to design and manufacture solid oral drug products, and solutions for such challenges are examined.
The use of various documents and guides by the International Pharmaceutical Excipients Council can facilitate the flow of information among excipient manufacturers, distributors, and users.

Specially designed excipients, improvements in processing capabilities, and a growing understanding of the hot-melt extrusion (HME) process is increasing the use of HME as an approach for enhancing the bioavailability of poorly soluble drugs.
As contract API manufacturers, fine-chemical producers, and pharmaceutical companies prepare to attend Informex in Anaheim this month, the state of the industry shows improving conditions in certain sectors.

Siegfried Schmitt, a principal consultant with PAREXEL, discusses how the EU's Falsified Medicines Directive will affect US API production.
Process chemists employ a variety of approaches to improve yield, purity, and stereoselectivity.

The agency recommends to avoid the use of to dibutyl phthalate and di(2-ethylhexyl) phthalate in CDER-regulated drug and biologic products, including prescription and nonprescription products.
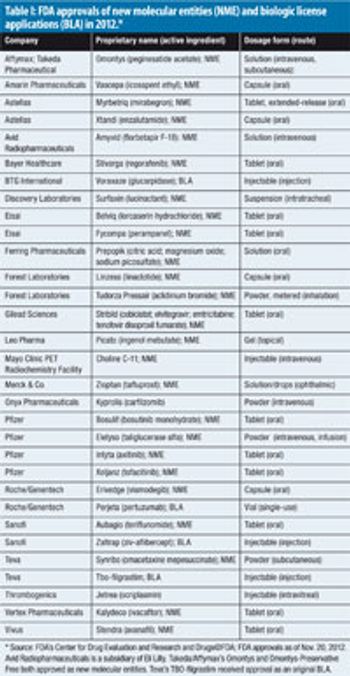
As 2012 comes to a close, Pfizer leads among Big Pharma companies for FDA approvals of new molecular entities and biologics.

Novartis announced that it has received FDA approval for a seasonal influenza vaccine produced in cell culture, the first seasonal vaccine produced by this method to be approved in the US.

Boehringer Ingelheim has entered into a non-exclusive technology and commercial license option agreement with BaroFold. Under the terms of the agreement, Boehringer Ingelheim will license BaroFold's pressure-enabled manufacturing technology (PreEMT).