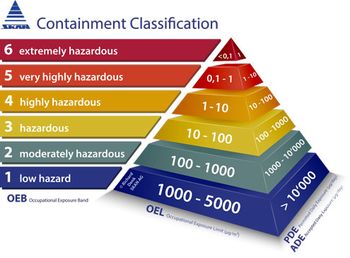
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

The article reviews strategies for firms with or without existing in-house capacities and the pros and cons for outsourcing bio/pharmaceutical development and manufacturing.

The agency cited the company’s Kansas facility with CGMP violations similar to problems found at other Hospira facilities.

Avella issued a nationwide recall of sterile products produced at the Advanced Pharma Houston location due to inaccurate labeling.

The company is voluntarily recalling one lot of Edex due to a lack of container closure integrity.

FDA sent a warning letter to Sato Pharmaceutical Co., Ltd. after inspectors found deviations in the facility’s aseptic processes.

The agency sent a warning letter to Resonance Laboratories Pvt. Ltd. after an inspection found possible contamination problems.

The company has voluntarily recalled all lots of of human chorionic gonadotropin because of a lack of sterility assurance.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses how to ensure sterility when manufacturing small-scale parenteral batches.

The agency finds repeated CGMP violations at Porton Biopharma, Limited.

The company issues a voluntary nationwide recall due to the presence of particulate matter in a single vial.

The company was cited by FDA for violations of sterile processing GMPs.

Airlocks, gowning rooms, and transition spaces have different uses and should be considered separately in cGMP pharmaceutical facility design.

Wockhardt Limited received a warning letter from FDA for CGMP violations.

Honeywell has launched a new range of gloves, Picguard Urban, which offers protection against needle stick and puncture.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the assessment of risk in the processing of intravenous injectable drugs.
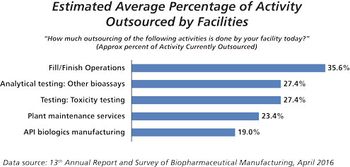
This key bioprocessing segment is expecting continued growth

Wells Pharmacy Network is voluntarily recalling all of its products due to sterility concerns.

FDA sent a warning letter to an Illinois compounding pharmacy for violations of the Federal Food, Drug, and Cosmetic Act.
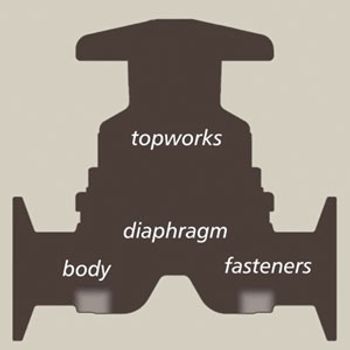
A process-specific preventative maintenance program improves productivity and reliability.

Visual inspection of parenteral vials is the first step in a root cause investigation.

Evonik’s parenteral drug delivery and commercial drug product manufacturing in Alabama passes EU GMP inspection.
Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?

Fresenius Kabi will add to its generic, sterile injectable manufacturing at its Melrose Park, Illinois site.
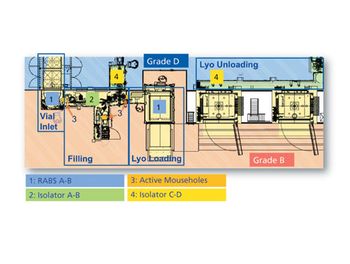
The design of Baxter BioPharma Solutions’ aseptic filling lines provides a case study in customizing containment systems for multi-product lines