
Gamma-stable fluoropolymers are an alternative material for single-use bags and assemblies in biopharmaceutical manufacturing.

Gamma-stable fluoropolymers are an alternative material for single-use bags and assemblies in biopharmaceutical manufacturing.

FDA issued a warning letter to the Worthing, UK facility for cross contamination and microbial contamination cGMP violations.

Teligent is expanding its manufacturing and R&D complex in New Jersey.

Early planning for the integration of clean-in-place systems for equipment cleaning is key.

Aseptic spray drying provides an alternative to lyophilization as an enabling stabilization technology for parenteral biologic formulations.

The agency cited the company for sterile manufacturing violations.
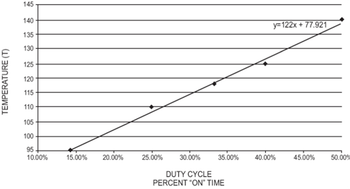
Preheating pinch valves prevents drift in the volume of liquid dispensed.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.

Intact enables aseptic filling in non-classified environments, maintains sterility throughout the process, and reduces costs, timelines, and contamination risks.

Non-destructive container closure integrity testing allows 100% inspection of all ampoules, syringes, vials, and cartridges.

The Matrix Alliance was formed by Vanrx Pharmasystems with inaugural members ARaymond Life, Daikyo Seiko, Datwyler Group, Ompi, Schott AG, Schott Kaisha and SiO2 Medical Products.

The agency cited Emcure Pharmaceuticals with CGMP violations.

Revised versions of ISO 14644 adopt changes to sampling procedures and monitoring plans for cleanrooms.

The Class 100 cleanroom oven from Grieve is used for a variety of heat processes such as sterilizing, depyrogenation, curing, and drying workloads.

Manufacturing of antibody drug conjugates requires high-containment solutions, such as high-performance aseptic isolators.

Regular removal of residues from disinfectants and sporicidals is important for improved aesthetics and safety in cleanrooms.

When implementing disposable technology for aseptic processing, considerations include material compatibility, material sourcing, facility layout, and training.

Sensaphone's monitoring system provides low-cost, 24/7 remote monitoring of unattended freezers and coolers.
Parenteral packaging of the future will include more automated lines, ready-to-fill packaging formats, and supply-chain transparency.

The revised USP Chapter 1207 gives best practices for obtaining reliable data in container closure integrity testing.

Collaboration between users and suppliers to increase understanding, clearly define user requirements, and mitigate risk is facilitating acceptance by industry and regulators.

Microorganism lethality requirements for process validation must always be balanced with the need to protect product integrity and patient safety.

All openings and potential apertures for air penetration must be considered when designing a cleanroom so that the HVAC system can maintain the desired negative or positive pressure.

Problems in an induction-sealing process, such as untorqued or crooked caps, can be identified and corrected in real time using dynamic thermal imaging.

Manufacturers of parenteral drugs face challenges to increase efficiency, control particulates, control extractables and leachables, and eliminate product/package interactions; new containers and packaging equipment offer increased options.