
The company is recalling several injectable products because of possible microbial contamination.

The company is recalling several injectable products because of possible microbial contamination.

EDQM held a symposium on microbiology to gather industry feedback on alternative testing methods for microbiological control and sterilization processes.

Water shortages, testing expenses, and effluent treatment are important issues for pharma manufacturers.

Xellia Pharmaceuticals invests US$25 million in its Copenhagen, Denmark site to expand sterile manufacturing of products for treating antimicrobial resistant infections.

Groninger will showcase its FlexPro 50 line for small batch production at CPhI Worldwide 2017, and present on up- and downstream processes with freeze-dryer developer and manufacturer Martin Christ.
Manufacturers introduce innovations in glass and plastic packaging for injectables.

This article discusses fully automatic inspection of glass and plastic containers and factors that affect particle detection rate.

The Steris VHP DC-A atmospheric pass-through chamber is designed for aseptic drug manufacturing and laboratory research.

Fresenius Kabi broke ground on a previously announced $250 million expansion of its Melrose Park, IL, manufacturing facility.

The FDA commissioner made a statement about the agency’s efforts to ensure patient access to safe compounded medicines.

Ajinomoto Althea’s Optima VFVM 7000 aseptic fill/finish line supports a range of drug substance APIs.

FDA sent a warning letter to Maple Rose Enterprises, Inc., dba Pencol Compounding Pharmacy, citing the company for violations of sterile manufacturing practices.

FDA sent a warning letter to Sage Products, Inc. regarding CGMP failures involving test methods, sampling, and sterilization procedures.

The agency cited the Italian company for aseptic processing failures.
Oral solid-dosage and parenteral drug manufacturing equipment and systems have made great strides in safety and efficiency.

Pharma company consolidation and outsourcing led to a de-emphasis of manufacturing and reduced investment in new technologies and facilities.

Advancements in cell culture and protein technology have opened the door for new therapies.

Fagron Sterile Services has voluntarily recalled three lots of Succinylcholine Chloride 20mg/mL 5mL syringe to the hospital/clinic level.

The need for flexibility and higher quality are driving advances in parenteral manufacturing and fill/finish equipment.

The company has voluntarily recalled Clindamycin Injection USP ADD-Vantage Vials to the hospital/retail level because of a lack of sterility assurance.

FDA sent a warning letter to drug compounder DCA, Inc. dba Beacon Prescriptions for failing to ensure sanitary conditions.
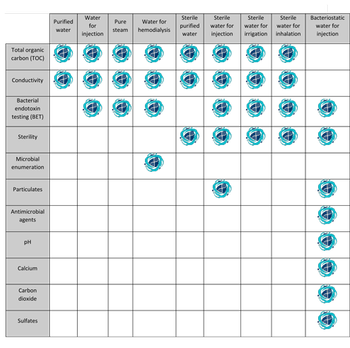
USP describes analytical and microbiology testing required for different types of pharmaceutical water.

FDA sent a warning letter to Pharmaceutic Labs, LLC for deficiencies in producing sterile drugs.

Robotic fill/finish systems reduce human intervention, improve flexibility, and allow more gentle handling of containers.

The type of water for pharmaceutical use is determined by USP testing.