
The STERIS ProKlenz ONE alkaline cleaner now has a label performance claim for biofilm removal, in addition to the product’s existing label as a disinfectant and virucide.

The STERIS ProKlenz ONE alkaline cleaner now has a label performance claim for biofilm removal, in addition to the product’s existing label as a disinfectant and virucide.

Kimberly-Clark Professional added the Kimtech A5 Sterile Integrated Hood and Mask XL for head shapes, sizes, and hairstyles that pose a challenge to standard aseptic gowning for cleanroom operators.
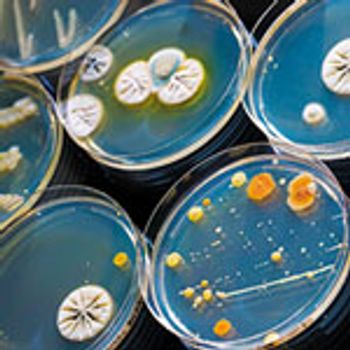
Microbial identity data can be critical for determining contamination sources.

Collaborative robots work beside laboratory employees to improve efficiency in pharmaceutical research and quality control labs.

The agency is developing a new way to assess, record, and report data from surveillance and preapproval inspections of sterile drugs.

Collaborative robots work beside laboratory employees to improve efficiency in pharmaceutical research and quality control labs.

The contract development and manufacturing organization expanded its analytical chemistry suite and added a new office in Boston, MA.

FDA sent a warning letter to Lernapharm (Loris) Inc. detailing the company’s lack of procedures to prevent microbiological contamination.

De Lama’s DLVHP/DE pass-box decontamination unit uses vacuum hydrogen peroxide sterilization.

Lonza’s new PyroTec Pro Robotic Solution provides a fully automated workflow for endotoxin detection.

Watson-Marlow Fluid Technology Group added a new actuator suitable for applications where reduced weight is a concern.

This article focuses on applying new and traditional techniques to design a cleaning process, ensure the surfaces are clean, and develop rinse solution analysis to continuously monitor cleaning performance.

Single-use technologies, modular systems, and robots are on the rise.

Automated robotic arms manipulate nested trays and containers inside closed aseptic filling systems.

Why shouldn’t biopharmaceutical manufacturers be able to leverage standardized construction practices to improve time to market?

Pfizer will invest nearly half a billion dollars to build a sterile injectable facility in Michigan.

The agency has issued a warning letter for cGMP violations at a drug manufacturing facility in Ahmedabad, India, that Baxter gained through its acquisition of Claris Injectables.

Both parties have agreed to recognize each other’s inspection of manufacturing sites for a broader range of medicines, including sterile products, APIs, biologics, and vaccines.

The author reviews current approaches to sterile containment and compares several sealed transfer and barrier techniques, including isolators, restricted access barrier systems, and split butterfly valve technology.

Vac-U-Max received “atmosphères explosibles” or equipment for potentially explosive atmospheres (ATEX) approval for three product ranges of compressed-air-powered vacuums.

Automated robotic arms manipulate nested trays and containers inside closed aseptic filling systems.

Flow imaging microscopy can be used to identify particulates and their sources.

PDA reviews an industry survey of concerns and best practices regarding the EU GMP’s required PUPSIT test for filters used in sterile filtration.

Rommelag will showcase its blow-fill-seal technology that collectively offers container production, aseptic filling, and container closure.

Optima will also display the freeze dryer CS and the sterility test isolator STISO at the show.