
Mylan received a warning letter citing violations at its Agila Specialty Formulation Facility, Sterile Products Division, and Onco Therapies Limited sites in India.

Mylan received a warning letter citing violations at its Agila Specialty Formulation Facility, Sterile Products Division, and Onco Therapies Limited sites in India.
Within the past few years, key players have left the sterile manufacturing business. Can new technology and investment revitalize this critical market?

Teva Parenteral Medicines initiates voluntary nationwide recall of select lots of Adrucil due to particulate matter.

Baxter has recalled of one lot of IV solution due to the potential for leaking containers, particulate matter, and missing port.

Walker Barrier Systems extended-width mobile cleanroom provides 1350 square feet of workable space.

Bill Hartzel, Director Strategic Execution, Advanced Delivery Technologies at Catalent Pharma Solutions, spoke with Pharmaceutical Technology about blow-fill-seal for aseptic processes.

Presenters at IVT's Microbiology Week discussed best practices and recent guidance publications for microbial control in sterile and non-sterile pharmaceutical processes.

Experts at Eppendorf discuss common challenges in cell culture and share insights on possible solutions.
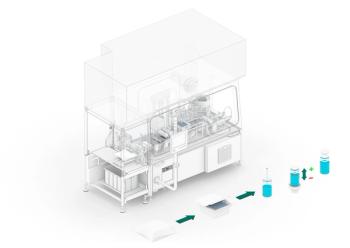
Groninger has developed FlexPro 50, an isolator system for aseptic processing of nested syringes, cartridges, and vials. FlexPro 50 is capable of achieving outputs of up to 5000 containers per hour.

Bosch’s market launches at the show include a new bioreactor for laboratory-scale API development, a water-for-injection unit, and a new generation of pure steam generators with high yields.

Telstar has developed an innovative in-line vial management system for recognition and marshaling of vials in automatic loading and unloading systems.

Machine manufacturer IMA is showcasing a number of technological advances for the processing and packaging of pharmaceutical products at ACHEMA 2015.

FDA cites 17 observations including air handling, quality control, and deficient microbial monitoring at NIH’s Clinical Center Pharmaceutical Development Section.

Careful choice of wash-water parameters and attention to water quality and basket loading are important for optimal cleaning.
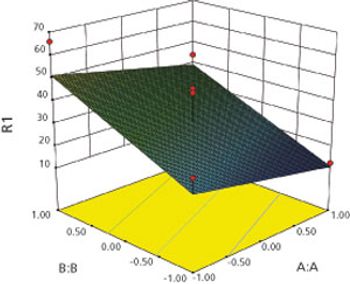
Box-Behnken modeling was used to optimize a resinate complex, to mask the taste of levocetirizine dihydrochloride and montelukast sodium in orally disintegrating tablets.

Grand River Aseptic Manufacturing named one of Michigan 50 Companies to watch.

OXY-CAPT brings together properties of plastic and glass containers through the combination of oxygen absorption and cyclic olefin copolymer (COP) high barrier layers.

Symbiosis Pharmaceutical Services is now offering a new bulk lyophilization (freeze drying) service in response to increasing demand in the manufacture of bulk intermediates and APIs by lyophilization.

This article looks at data gathered from several studies of a widely marketed chlorobutyl rubber formulation used for vial stoppers and prefilled syringes.

This study evaluates the impact of controlled nucleation on the ability to optimize a lyophilization cycle for a monoclonal antibody formulation.

Traditional sterility testing methods can take 14 days or longer to complete, so a growing number of pharmaceutical manufacturers and quality control

The use of disposables requires careful consideration and planning.

Total cost of ownership, including operating costs, must be considered when deciding between flexible and traditional cleanroom systems.

Aging facilities and inadequate quality systems create drug shortages. Preventing them will require harmonized regulations, and management support for continuous improvement and innovation.

The company voluntarily recalls select lots of Adrucil due to particulate matter.