Drug developers must understand the complex bioanalytical assays for cell- and gene-therapy drug development programs and ensure that partners have the specialized expertise needed for complex therapeutic classes.

Drug developers must understand the complex bioanalytical assays for cell- and gene-therapy drug development programs and ensure that partners have the specialized expertise needed for complex therapeutic classes.

This injection formulation of USP-grade leucovorin calcium is now available in the United States.
This article demonstrates how qualitative physical attributes testing can be used to characterize soft gel capsule rupture/disintegration during rectal administration.

Virpax’s Patch-in-a-Can technology delivers pain medication using a metered-dose spray film.

Drug and adhesive formulation are crucial to the development of microneedle patches for pharmaceutical transdermal delivery systems.
Antibody-based drugs offer new mechanisms of action and greater specificity.
Using training devices may ease patient anxiety about using autoinjectors and prefilled syringes, potentially leading to improved patient adherence.

ADC Biotechnology will invest downstream formulation, fill/finish capabilities, and Lock-Release conjugation technology.

Copley Scientific-a manufacturer and provider of test instrumentations for pharmaceutical dosage forms-has officially opened its newly expanded headquarters in Colwick, United Kingdom.

This paper describes how the concept of acceptance value can be redefined to remove bias and more closely reflect quality targets.

Demand for highly potent APIs continues to rise.

Survey results and record attendance show positive signs for various bio/pharma regions.

Cambrex will create a new center of excellence for API process development and clinical supply at its High Point, NC facility and expand its API manufacturing facility in Italy.

At CPhI Worldwide 2018, Tom Wilson, vice president of Contract Manufacturing Operations for Pfizer Global Supply and Pfizer CentreOne Contract Manufacturing, will discuss best practices for meeting manufacturing challenges of compliance as requirements shift.

Funding from Innovate UK will be used by Medherant to support manufacture of transdermal patches for clinical trials.

Innovative technologies, such as drug-loaded devices and 3D printing, enable advances in implantable devices and other novel dosage forms.
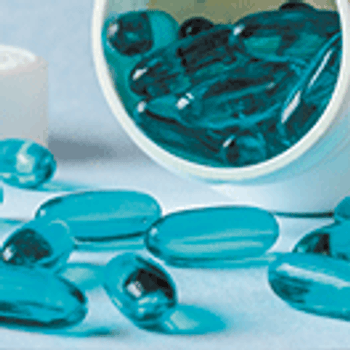
Lipid-based formulations offer a means of addressing the physicochemical and biological challenges of poorly soluble APIs.

Vetter chose a winner from four teams that worked for three months to develop ideas for applying digital technology to injectable pharmaceuticals.

3D printing is being explored as a manufacturing method for on-demand, personalized medicine.

As it investigates the root cause of an impurity discovered in valsartan, FDA extends its studies to APIs with similar synthesis processes.

Senomyx uses an approach, known as taste-blocking, to address the challenges of bitter APIs. Kenneth J. Simone, vice-president of Pharmaceutical Business Development, Senomyx, speaks with Pharmaceutical Technology about this technology.

Sharing know-how can help resolve common bio/pharma technical challenges.

Existing software tools cannot take into account the complexity of disease.
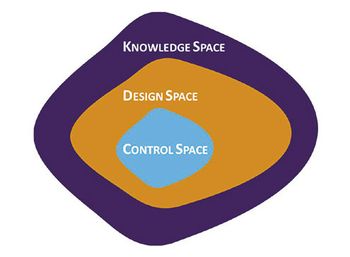
Using a QbD approach in the development and formulation of topical products will enable the drug developer to provide a robust control strategy for manufacturing.
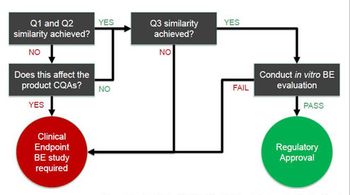
This article examines IVBE testing requirements for topical creams and explores some of the analytical techniques necessary.