
The 59th Pittsburgh Conference gathered more than 1000 exhibitors on its showroom floor this week.

The 59th Pittsburgh Conference gathered more than 1000 exhibitors on its showroom floor this week.

Chemical imaging of solid dosage forms has become a powerful analytical tool for the development of solid dosage forms.
The pharmaceutical industry has experienced a number of difficulties during recent years. Greater competition from generics (more than 60% of prescription drugs are supplied from the generic market) and increased gaps in the drug pipeline that result in acquisitions or strategic alliances has led to a feeling of uncertainty in the bio/pharma marketplace. There have also been changes in the marketplace with a shift from primary care to specialty drugs, the introduction of personalized medicine driving the need for biomarker/diagnostic technology and the introduction of biopharmaceuticals.

To ensure an effective treatment, a patient often must take equal doses of an active pharmaceutical ingredient (API) at regular intervals.
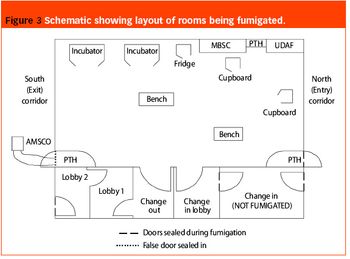
As part of a major project to design and build a new bulk vaccine antigen plant, the authors were asked to investigate and implement a suitable fumigation system for clean room decontamination. The facility was designed to handle and contain live influenza virus, and has clean room suites designed to containment levels CL2 and CL3 according to the Approved Code Of Practice and Guidance (ACOP, Control of Substances Hazardous to Health 4th Edition). From the outset, specific areas within the facility were identified as requiring fumigation and this formed part of the initial design brief.

We are currently experiencing a problem with one of our tablet lines. While the tablets appear white immediately after manufacture, after a time many of the tablets begin to take on a yellowish appearance. Could this be an issue that surface analysis could help resolve?
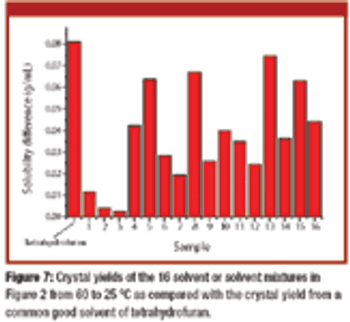
The authors propose extending initial solvent screening for a single-solvent system to the cocktail solvent screening of binary and ternary solvent mixtures.

Natural gums and mucilage are biocompatible, cheap, readily available, and represent a potential source of excipients. The authors examine the functionality of mucilage extracted from the leaves of Hibiscus rosa-sinensis Linn as an excipient in a sustained-release tablet formulation.
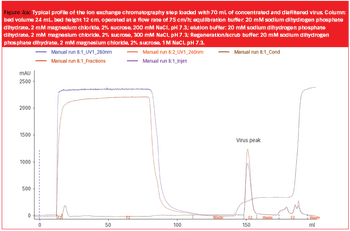
The use of viral vectors as gene delivery and vaccine vehicles has developed rapidly during the last two decades owing to several viral properties. Viruses can infect cells efficiently, often have a broad tissue tropism and can achieve very high levels of either stable or transient transgene expression. Furthermore, their intrinsic immune-stimulatory properties can have adjuvant effects during the treatment of cancer or infectious disease and, importantly for manufacturing scale-up, some viruses can be grown to very high titre (.1012 particles/mL). The development of robust production procedures is essential to move therapeutics that utilize viral vectors into clinical trials, and to make them cost effective for market supply. Here, we describe some of the aspects of production that must be considered and optimized when producing virus vectors on an intermediate or large scale. By drawing examples from our experience of vector production, we show that upstream and downstream processes must be designed..

Merck & Co. initiated a voluntary recall of 11 lots of its Haemophilus influenzae type B vaccine, Pedvaxhib, and two lots of its combination Haemophilus influenzae type B/ hepatitis B vaccine, Comvax.

The US Food and Drug Administration issued a rule that clarifies its requirements for current good manufacturing practices for aseptic processing, water standards, and verifications standards.

To keep up with applications, FDA promotes new testing and administrative methods.

Individualized dosing for specific patient needs has been the goal of medical and pharmacotherapy specialists since they first envisioned pharmacogenetic evaluation. With the measurement of individual levels of metabolism, the optimum dose can be calculated for each individual patient.
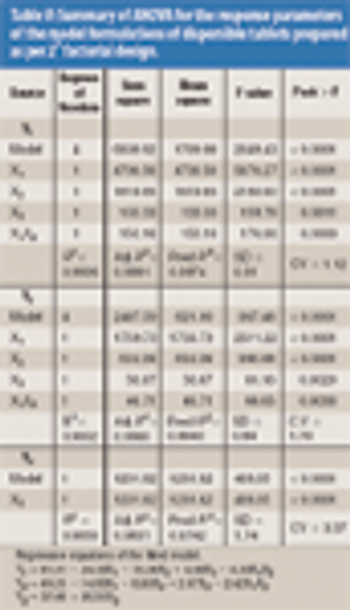
The authors analyzed the effects of complexation as well as the levels of ammonium bicarbonate and crospovidone on tablet wetting time (WT), disintegration time (DT), and percent dissolution efficiency at 60 min (%DE60).

Incorporating quality and economy into downstream purification processes can expedite first-in-human clinical trials, product licensure and technology transfer. Experience in the chromatographic purification of biopharmaceuticals enables the use of downstream processing heuristics to produce target molecules in cost-effective processes suitable for regulatory scrutiny.

Sanofi-aventis entered into an agreement with Chinese authorities allowing Sanofi to construct a vaccine-manufacturing plant in Shenzhen municipality.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Market demand for cytotoxic drugs is leading CMOs to expand their API manufacturing and formulation services.
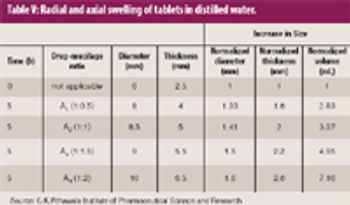
Natural gums and mucilage have been widely explored as pharmaceutical excipients. The goal of this study was to extract mucilage from the leaves of Aloe barbadensis Miller and to study its functionality as an excipient in pharmaceutical sustained-release tablet formulations.

Vaccines are needed against old and new infectious disease threats - polio and other childhood illnesses, bioterrorism and pandemic flu. They are also emerging for cancer immunotherapy and for treating addiction. While vaccines are among some of the most successful biotech products, their large-scale manufacture involves some special demands, such as maintaining a good working cell bank and gearing up for production on an 'as needed' basis.

The crystalline structure of pharmaceutical solids can sometimes be altered during processing. X-ray powder diffraction and near infrared spectroscopy can be used to determine the amorphous and crystalline content of a model substance. The two techniques' precision, accuracy, detection limit and the speed of analysis are compared.
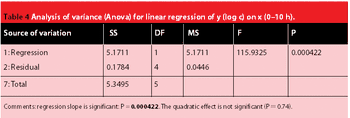

The pharmaceutical market is undergoing a major transformation with companies moving more and more toward the generics business, according to a new report.

Ranbaxy received full market approval from the US Food and Drug Admnistration for its anti-infective agent ?Clarithromycin? oral suspension.