
A groundbreaking ceremony was held in Rahway, NJ for Merck’s new FLEx facility that incorporates G-CON’s prefabricated cleanroom units.

A groundbreaking ceremony was held in Rahway, NJ for Merck’s new FLEx facility that incorporates G-CON’s prefabricated cleanroom units.

In this collaboration, AbCellera will apply its expertise to generate panels of antibody candidates for Gilead to evaluate.

The acquisition will boost Biogen’s gene-therapy pipeline with the addition of two mid- to late-stage clinical assets and preclinical programs.

Cambrex has announced the completion of its new facility at its Karlskoga site in Sweden that combines new laboratories for process and analytical development.

CPI, has supported a project focused on developing and integrating novel recombinant prokaryotic lectins (RPLs) into a biosensor-based platform.

Excipients and new processing techniques can make a real difference in the development of highly potent therapies.

Nanobiotix has launched Curadigm, a spinoff company that will specialize in developing a nanotechnology platform for healthcare applications.

MilliporeSigma awarded its Advance Biotech Grant to three US companies that are focused on traumatic brain injury, vaccine development, and chronic pain.

New offering from Catalent targets integrated development and manufacturing of biologic drugs.

Eli Lilly is set to acquire exclusive worldwide rights for potential non-opioid treatment CNTX-0290 from Centrexion Therapeutics in a deal potentially worth $997.5 million.

Researchers at the University of Sheffield, Sheffield, UK, have discovered and developed a new compound that can kill antibiotic-resistant bacteria, including Escherichia coli.

Expanded controlled substance capabilities enables Particle Sciences to develop and manufacture Schedule I–V drugs.

The companies will develop a new generation of biotherapeutics from cell-line development through to GMP manufacturing.

The guidance discusses the design and evaluation of comparative analytical studies used to support the biosimilarity of a proposed therapeutic protein product to a reference product licensed under section 351(a) of the Public Health Service Act.

AbbVie grants Boehringer Ingelheim a non-exclusive license to its intellectual property for Humira (adalimumab) in the United States.

Regulatory, cultural, and technical differences between drug and device development can pose challenges to pharmaceutical combination product development.

The company announced plans to construct a 1.3 million-ft2 integrated manufacturing center in Chengdu, China.

Locate Bio has been granted additional investment to the tune of £2 million (US$2.6 million) for the expansion of its cell and gene therapy pipeline.

The acquisition provides Valby, Denmark-based Lundbeck with a discovery platform and a US-based research hub.
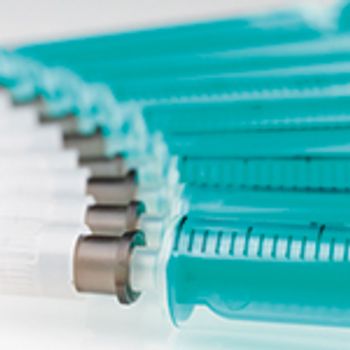
Empty and prefilled syringes must pass a range of quality control tests.

Dyadic International has announced its collaboration agreement with Serum Institute of India for the development and manufacture of up to 12 antibodies and vaccines using Dyadic’s C1 gene expression system.

The guidance will assist sponsors in demonstrating a proposed therapeutic protein product is interchangeable with a reference product.

Lonza will provide development and manufacturing services to two of Alector’s neurodegeneration drug candidates.

Samsung BioLogics will provide contract development organization services to drug developer GI Innovation.

The companies have entered into a sub-licensing agreement to develop products based on Dyadic’s C1 expression platform and Alphazymes’ enzyme technology.