
Comecar will provide cell culture equipment for biopharmaceutical company CO.DON AG's new production site in Leipzig, Germany.

Comecar will provide cell culture equipment for biopharmaceutical company CO.DON AG's new production site in Leipzig, Germany.

Clover Biopharmaceuticals will use GE's FlexFactory biomanufacturing platform with single-use bioreactors to produce innovative and biosimilar fusion protein products in China.

Lonza Pharma & Biotech's's Modular Automated Sampling Technology (MAST) platform, which allows for the collection of up to 10 sterile sample sources for automated analysis in multiple analytical devices, won the CPhI Pharma Award 2017 for Excellence in Pharma: Bioprocessing.

GE Healthcare will equip Cellular Biomedicine Group's cell therapy manufacturing facility in Shanghai, China, with its FlexFactory single-use platform, designed to speed up cell therapy manufacturing timelines.

The Industrial Internet of Things and new tools such as smart glasses can improve training and daily tasks for pharmaceutical manufacturing operations.

Sartorius Stedim Biotech will equip Abzena’s contract development and manufacturing facilities in Bristol, PA, and San Diego, CA, with single-use equipment and scale-up technologies.

The Tamper Evident Labeller from LSS Labelling Systems Scandinavia is compliant with the European Falsified Medicines Directive.

In a roundtable Q&A with biopharma executives, the vulnerability and challenges of dealing with extractables and leachables in single-use bioreactor bags are explored.

Proper selection and installation optimizes fluid system performance.

Closures that protect solid-dosage drugs and the capping equipment that applies them have new product-protecting features.

The Wilden Quattroflow QF10k size pump from PSG, a Dover company, has been designed to fill the gap between the existing QF4400/5050 and QF20k pump sizes.
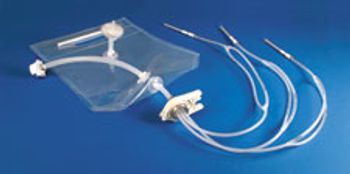
Sterile-molded filling assemblies from AdvantaPure are suited for single-use vial and syringe filling.

MilliporeSigma will collaborate with IPS and G-CON to offer end-to-end, turnkey, modular MAb manufacturing.

BioTek Instruments has released a second edition of its BioSpa software that now offers users a simplified but effective interface for kinetic imaging or detection workflows.

MJR PharmJet's MicroJet Reactor technology is a continuous process for producing nanoparticles with tightly controlled particle size and particle size distribution.

The Quattroflow QF10k size pump from PSG, a Dover company, has been added to its line of quaternary, four-piston diaphragm pumps.

The new 30,000-L, $150-million biologics manufacturing facility in Wuxi, China, quintuples the company’s existing manufacturing capability.

PTI moved to a new facility in Belgium that will house its headquarters and a R&D center for tablet dedusting, capsule polishing, and testing technology.

Fette Compacting and Glatt have joined forces to develop integrated solutions for the continuous manufacturing of oral solid-dosage drug forms.

SciLog Select Go Single-Use Assemblies from Parker Hannifin uses an assortment of validated parts and assemblies to build needed devices for biopharmaceutical manufacturing.

The company’s new modules offer scalable single-pass diafiltration and were exclusively showcased during its Leadership Forum series in Westborough, MA.

The companies have established a joint laboratory to develop full continuous processing to manufacture high yields of monoclonal antibodies at reduced costs.

A pneumatic cover lift for the Witte fluid bed dryer raises the cover for cleaning and inspection.

Alfa Laval rotary jet heads can be installed in small tank openings to replace static spray ball or rotary spray heads.

Industry experts were honored for business, scientific, and social contributions.