A process-specific preventative maintenance program improves productivity and reliability.

A process-specific preventative maintenance program improves productivity and reliability.

The platform combines an expression system with equipment and process controls to enable rapid development and scale-up of robust titers.

GE and Sealed Air extend their long-term collaboration to develop single-use films purposefully constructed for bioprocessing applications.

The company announced that it will be expanding its portfolio of single-use vessels for fermentation.

Irvine Scientific’s new product range includes chemically-defined, serum-free media, to increase productivity of viral vectors and recombinant proteins in suspension cultures.
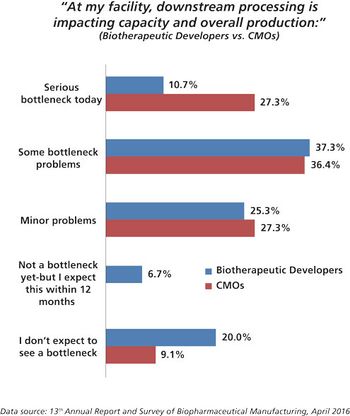
CMOs are working hard to improve performance by investigating new technologies for filtration and purification.
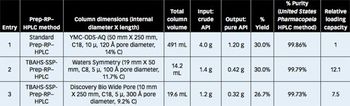
This paper describes a unique Prep-rP-HPLC technique that uses a C-18/C-8 derivatized silica coated with a hydrophobic quaternary ammonium salt or quaternary phosphonium salt that acts as an additional/surrogate stationary phase (AsP/ssP).

Gamma-stable fluoropolymers are an alternative material for single-use bags and assemblies in biopharmaceutical manufacturing.

Early planning for the integration of clean-in-place systems for equipment cleaning is key.

The Pharmacopoeial Discussion Group approved monographs and plans to harmonize several others.

Pfizer broke ground at its Andover, Massachusetts campus on a clinical manufacturing facility for complex biologics and vaccines.

The company will be exhibiting at the 2016 International Fuel Ethanol Workshop and Expo.

Alvotech prepares for commercial biosimilar production in new facility with single-use bioreactors in Reykjavik, Iceland.

Customized puresu single-use fluid path assemblies from Watson-Marlow Fluid Technology Group incorporate tubing and BioPure components and are supplied ready to use.

Effective harvesting and purification processes play an essential role in ensuring that biopharmaceutical manufacturing processes provide biologic drug substances with uniform and consistent properties.
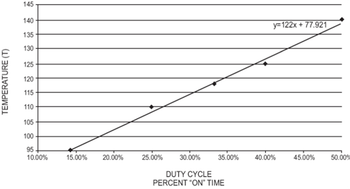
Preheating pinch valves prevents drift in the volume of liquid dispensed.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.
Process analytical technology paved the way for continuous manufacturing.

W.R. Grace & Co. will sell its chromatography product lines, which includes chromatography instruments, columns, and other related products.

Packaging technologies for serialization, parenteral products, and solid-dosage forms were displayed at INTERPHEX 2016.

In this article, the authors look at the limitations of the validation for a single-use shipping system and provide a perspective on what shipping validation means.

Fogg Filler’s enclosures for bottle-rinsing machines have improved fume containment and doors with lift-off hinges for maintenance.

MilliporeSigma expands its Carlsbad, California-based GMP capacity for viral and gene-therapy products by nearly 90%.

Bioprocess operations-from cell line selection to final filtration-can influence the consistency and purity of biologic drug substances.

MilliporeSigma, the North American life-sciences business of Germany’s Merck KGaA, added a 2000-L single-use bioreactor to its facility in Massachusetts.