
Analytical laboratory equipment adapts for new realities of downsizing, outsourcing, and speed demands.

Analytical laboratory equipment adapts for new realities of downsizing, outsourcing, and speed demands.

The IH-TMS Tool Management System from I Holland monitors tool rotations, tooling inventory, and maintenance.

The advanced capillary electrophoresis system, Maurice, from ProteinSimple is used for the quantitative analysis of identity, purity, and heterogeneity profiles of biopharmaceuticals.

High Speed Dispersers from Ross feature a National Electrical Manufacturers Association (NEMA) 7&9 Operator Panel and grounding systems.


The Class 100 cleanroom oven from Grieve is used for a variety of heat processes such as sterilizing, depyrogenation, curing, and drying workloads.
The authors provide their perspectives on shipping validation.

Tablet presses require regular inspection and maintenance to prevent premature wear and tableting problems.

National Bulk Equipment’s material handling system for non-free flowing materials can be specified to meet cGMP cleanability standards.

Manufacturing of antibody drug conjugates requires high-containment solutions, such as high-performance aseptic isolators.

When implementing disposable technology for aseptic processing, considerations include material compatibility, material sourcing, facility layout, and training.

Scale-down modeling is instrumental in supporting the development of downstream biopharma manufacturing processes.


Today’s analytical laboratory equipment reflects the realities of downsizing, outsourcing, and the need for speed.

Today’s analytical laboratory equipment reflects the realities of downsizing, outsourcing, and the need for speed.

The Alfa Laval Safety Valve, a true spring-loaded safety valve, is designed to protect both equipment and people.

Mass Spec Lab, a privately owned analytical company, announced its official launch on Dec. 28, 2015 in Southern California.

Collaboration between users and suppliers to increase understanding, clearly define user requirements, and mitigate risk is facilitating acceptance by industry and regulators.

Millipore Express PHF hydrophilic, sterilizing-grade filters from EMD Millipore offer faster flow rates to speed the process or reduce the process footprint.

Grand River Aseptic Manufacturing expands disposable technology capabilities at aseptic facility.

PSL has installed several advanced contained filtration and drying facilities as part of an expansion of a pharmaceutical manufacturing plant in Singapore.
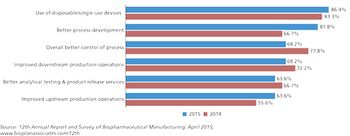
Better process development is creating industry benchmarks for bioprocessing.

GE Healthcare Life Sciences and Emerson Process Management collaborate in biopharmaceutical manufacturing processes.

GE Healthcare's KUBio prebuilt modules were shipped from Germany to JHL Biotech in Wuhan, China.

Problems in an induction-sealing process, such as untorqued or crooked caps, can be identified and corrected in real time using dynamic thermal imaging.