
The need for flexibility and higher quality are driving advances in parenteral manufacturing and fill/finish equipment.

The need for flexibility and higher quality are driving advances in parenteral manufacturing and fill/finish equipment.

The company added new products to its InfinityLab LC Series which will be showcased at HPLC 2017 in Prague.

The past several months have seen new product releases and updates made to already available laboratory equipment.

Single-use systems demonstrate advantages over stainless-steel systems for biopharmaceutical manufacturing facilities.

A multi-pronged approach to raw materials testing can help mitigate the risk of future contamination events.

The decision to use disposable bioreactors is now driven by commercial rather than technological considerations.

Avid, a wholly owned subsidiary of Peregrine Pharmaceuticals, will upgrade its Myford, California clinical and commercial manufacturing facility with multiple Mobius 2000-L single-use bioreactors from MilliporeSigma, the companies announced on May 1, 2017.

Bosch’s new system for the production of water for injection uses membrane processing and ultrafiltration.
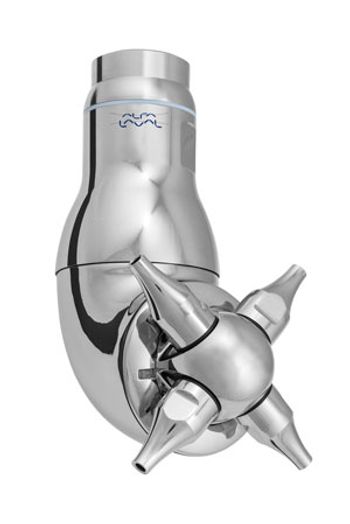
The Alfa Laval TJ40G tank cleaning machine with a four-nozzle rotary jet head cleans tanks quickly with less water.

Watson-Marlow Fluid Technology Group’s ASEPCO Weirless Radial diaphragm valves eliminate dead legs and product entrapment.

The process control and automation requirements of single-use systems differ from those of stainless-steel equipment.

QF20kSU single-use pumps from Quattroflow are used for applications requiring gentle product handling, high containment, low pulsation, purity, and cleanability.

Parenteral packaging will be well-represented at INTERPHEX, especially technologies associated with fill/finish of ready-to-use vials, cartridges, and syringes.
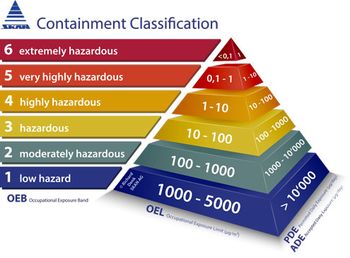
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

The pharmaceutical industry now has a way to accurately evaluate and compare dust collection systems that are self-cleaning

Pump systems must be designed to meet the needs of specific processes, including preventing cross-contamination and damage due to shear forces.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.

GEA’s ConsiGma continuous tableting line combined with Siemens’ automation and Sipat data management systems enables continuous manufacturing.

The authors describe the development and validation of a highly sensitive point-of-use pressure decay test.

EMD Millipore’s Mobius Power MIX 2000 can mix buffers, culture media powders, and other challenging materials.

Recipharm is investing more than EUR1.2 million to enhance its small-scale GMP API development and manufacturing capabilities in Paderno Dugnano, Italy.

Consider the purity of exhaust air emitted by vacuum pumps in addition to the purity of compressed air used in the pharmaceutical process.

Meissner’s QuaDrum rigid outer containers for its single-use assemblies can be used with standard, access, or retaining lids.

Novasep’s BioSC Lab chromatography is a flexible equipment that enables operations from one column up to six columns in batch, parallel batch, or continuous processing with all media and membrane types.

A PESU membrane is now available for Sartorius Stedim Biotech Sartocon benchtop and production-scale filtration assemblies.