
GlaxoSmithKline (London), Novartis (Basel, Switzerland), sanofi Aventis (Paris), and Baxter International (Deerfield, IL) recently provided updates as to the development, manufacture, or shipment of pandemic (H1NI) vaccines.

GlaxoSmithKline (London), Novartis (Basel, Switzerland), sanofi Aventis (Paris), and Baxter International (Deerfield, IL) recently provided updates as to the development, manufacture, or shipment of pandemic (H1NI) vaccines.

Company and People Notes: GSK forms joint venture with China-based Jiangsu Walvax Biotech; Sigma-Aldrich appoints VP and board member; more...

AAPS President offers hope and solutions for the industry's challenging future.
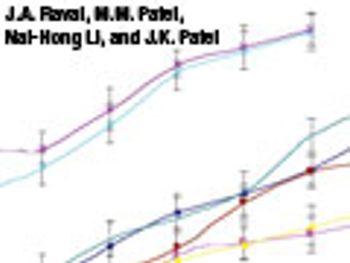
The authors investigated the effects of formulation and processing parameters on floating matrix-controlled drug-delivery systems.

The authors relay the outcome of a two-day workshop that brought together regulators and generic-drug industry representatives.

BIO supports recent Congressional action toward a 12-year data exclusivity period for innovators.

The nation's healtcare system needs an overhaul, but it has to be done right.

New nanotechnology-based delivery systems offer promise in drug delivery, particularly for anticancer therapeutics.

Brief pharmaceutical news items for October 2009.

The FDA issued a proposed rule to clarify the cGMP requirements applicable to combination products in the Federal Register.

Two vaccines manufactured by Novartis and GlaxoSmithKline (GSK) against influenza H1N1 have been recommended by the EMEA for marketing authorization.

Brown discusses the latest industry developments and trends.

Applying a moisture protective barrier to tablet cores can help prevent degradation caused by ambient moisture.

Formoterol presents formulators and manufacturers in the asthma and chronic obstructive pulmonary disease marketplace many challenges.

Company and People Notes: Boehringer Ingelheim will acquire Wyeth's animal health business; Amsterdam Molecular Therapeutics appoints CEO; more...

On September 25, the European Medicines Agency's (EMEA) Committee for Medicinal Products for Human Use (CHMP) recommended the authorization of two vaccines for use in Europe against the H1N1 influenza: GlaxoSmithKline's (GSK) Pandemrix and Novartis's Focetria.

A joint venture between a charity and a pharma giant has led to the creation of the Hilleman Laboratories, which will use a not-for-profit operating model to develop and deliver vaccines to low-income countries.

Company and People Notes: Wyeth and Ambrx form development pact; Elite Pharma appoints CEO and CSO; more...

The US Food and Drug Administration approved on Tuesday four H1N1 flu vaccines that demonstrated in clinical studies that a single dose produced a strong immune response in healthy adults after 8?10 days. But clinical trials of the vaccine are still underway on pregnant women and children, two groups that the Centers for Disease Control and Prevention (CDC) says are especially vulnerable to the H1N1 flu.

The FDA has approved four vaccines for the H1N1 influenza virus, while other companies, including UK pharma giant GlaxoSmithKline (GSK), have reported promising results from clinical studies with a single dose vaccine.

The US Food and Drug Administration has approved four vaccines for the H1N1 influenza virus, while other companies, including GlaxoSmithKline (GSK), have reported promising results from clinical studies with a single dose vaccine.

Company and People Notes: Sanofi Pasteur signs H1N1 vaccine deal with Brazilian government; Helsinn Group appoints CEO of US business; more...

Encap Drug Delivery (UK) has licensed a coating technology that targets the release of drugs to the colon.

Generic-drug companies are increasingly viewing the development of controlled-release formulations as a way of obtaining a competitive edge, according to a report published by Espicom Business Intelligence in August 2009.

Generic-drug companies are increasingly viewing the development of controlled-release formulations as a way of obtaining a competitive edge, according to a report published by Espicom Business Intelligence in late August 2009.