
The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

Also, WACKER expands Iowa facility; EMEA releases a Q&A document for PIPs; Metrics consolidates quality operations; more...
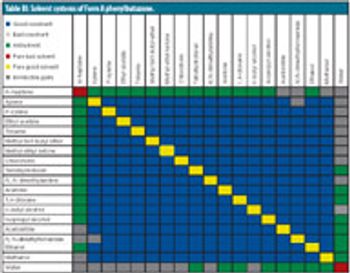
The authors describe the importance of a rapid and an abbreviated screening strategy in initial solvent screening. This article contains bonus online-exclusive material.
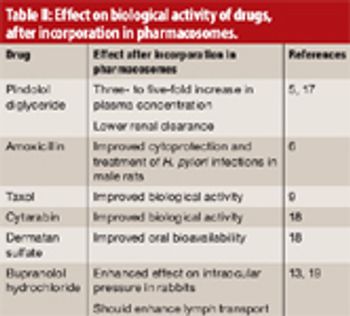
Pharmacosomes can pass through biomembranes efficiently and possess several advantages over traditional vesicular drug-delivery systems.

Misleading the public about their investments-be it money or medicine-is unacceptable.

CROs and CMOs expand to gain a piece of the market for clinical trial materials.

Follow-on biologics could unleash the potential of several industries and may even spark economic recovery.

It can take a lot of work to make sure nothing happens.

There may well be a pending revolution in biopharmaceutical expression systems. Nearly 50% of biomanufacturers today are demanding a whole lot more from their primary expression systems than they have during the past 30 years.
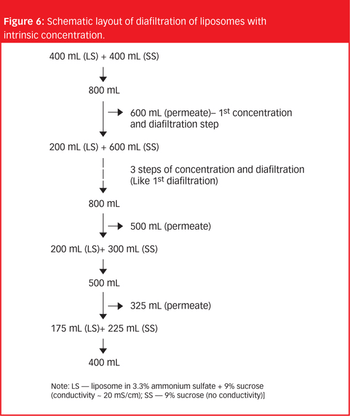
The importance of liposomes as an effective drug delivery system is well accepted in the pharmaceutical industry, but their handling remains a challenge.

The Question-Based Review (QbR) initiative of the Office of Generic Drugs has reached its second full year in 2009. Special from the Journal of Validation Technology.

The United States Department of Health and Human Services (HHS) this week placed an initial order with Sanofi Pasteur (Lyon, France) for a vaccine to fight influenza A (H1N1) infection.

Also, Johnson & Johnson acquires Cougar Biotechnology; NIH launches program for rare and neglected diseases; PPD restructures leadership positions; more...

Also, Oxford BioTherapeutics forms drug development pact with GSK; Avila Therapeutics names CEO; more...

According to a report from Greystone Associates in Amherst, New Hampshire, prefilled syringes are replacing vials both in terms of injections and sector revenue, and as this trend continues during the next four years, the number of tailored injectable drug products reaching the market is set to escalate.

Sanofi-aventis announced this week that it plans to construct a new vaccine-manufacturing center in Neuville-sur-Saône, France.

Also, Takeda to consolidate operations in Ireland; FDA to redesign website; Archemix names president and CEO; more...

Covance talks about the risks, challenges, and tests required to develop a pandemic vaccine.

Also, Oxford BioMedica forms collaboration with sanofi-aventis; FDA requires labeling changes for botulinum toxin producs; C. Richter King joins IAVI; more...

The president of BIO proposes the ingredients needed for industry growth.
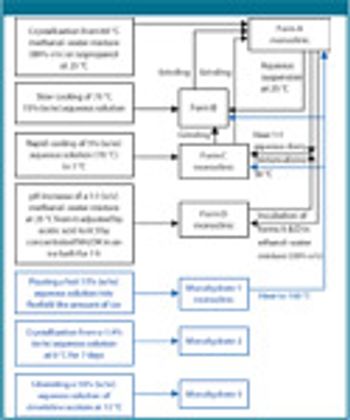
The authors describe the importance of a rapid and an abbreviated screening strategy by initial solvent screening in 20-mL scintillation vials.
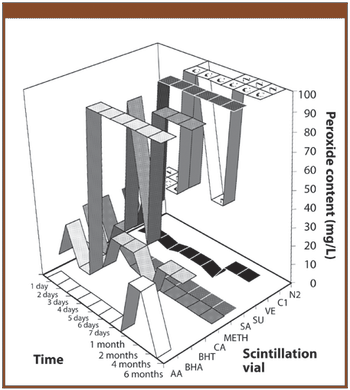
The authors investigate whether the addition of an antioxidant could be used to stabilize the solvent ethyl lactate by preventing the formation of peroxides

Brief pharmaceutical news items for May 2009.

Technology can solve enterprise-level problems.
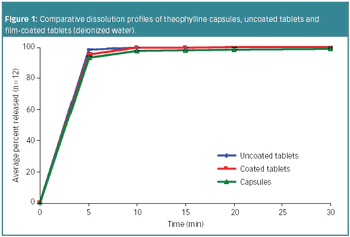
Based on formulation simplicity and blinding capability, hard gelatin capsules are preferrable compared with other oral solid dosage forms, including tablets, in the early clinical phases of drug development.