
Company and People Notes: Genzyme provides updates on enzyme replacement products and FDA complete response letter; Watson appoints VP of global operations; more...

Company and People Notes: Genzyme provides updates on enzyme replacement products and FDA complete response letter; Watson appoints VP of global operations; more...

Company and People Notes: 3M forms agreement with VaxInnate; TRIN Pharma appoints CEO; More...

The American Association of Pharmaceutical Scientists (AAPS) recognized researchers at the organization's 2009 Annual Meeting and Exposition in Los Angeles this week.

Novartis reported that German regulatory authorities approved its adjuvanted cell culture-based Influenza A (H1N1) 2009 monovalent vaccine, Celtura. The company also said it will acquire a stake in Chinese vaccines company Zhejiang Tianyuan Bio-Pharmaceutical.

The European Medicines Agency, the European Center for Disease Prevention and Control, and the Heads of Medicines Agencies issued a European strategy for H1N1 vaccine benefit–risk monitoring.

Leading experts from an upcoming conference on biosimilars by the Drug, Chemical, and Associated Technologies Association share their insights on the manufacturing and regulatory considerations for biosimilars.

In response to a request from the US Food and Drug Administration, the US Pharmacopeial Convention (USP) revised its standards for propylene glycol and sorbitol solution

Company and People Notes: sanofi aventis forms pact with Micromet; Laureate Pharma adds members to its business team.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.
The authors explain a process for moisture-activated dry granulation in detail and provide guidance for the selection of excipients and equipment.

FDA's Edwin Rivera-Martinez on dodging supply-chain challenges.
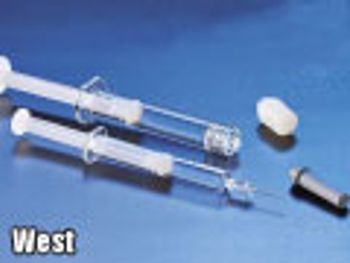
The authors describe the ways in which plastic prefilled syringes can be an alternative that provides consistent performance, protects drugs prone to degradation, and enhances patient safety.

The author describes how Merrion Pharmaceuticals reformulates parenteral drugs into tablets and capsules that are easier for patients to take and provide better bioavailability.

The author details the factors in formulation design, requirements in facilites and equipment, and validation criteria for aseptic formualtions.

A Conversation with Greystone Associates' George Perros. This article contains bonus online-exclusive material.

The author discusses the risks involved with aseptic processing, methods and tools used to identify and control risk, and regulatory guidelines relevant to the risk-management process.

GlaxoSmithKline (GSK) appointed 42 Technology (42T) to evaluate existing powder dispensing approaches that could be scaled for high speed manufacturing.

Chronotherapeutic drug delivery systems (CRDDS) have been recognized as potentially beneficial to the chronotherapy (timeoptimized therapy) of widespread chronic diseases that display time-dependent symptoms.

Mark Copley discusses the methods used for DPI testing and the challenges presented by the current regulatory framework.

An inhaler mouthpiece that optimizes drug delivery to the lungs and reduces the amount of wasted medication has been developed by US researchers.

The global API market has expanded from $69 billion in 2004 to more than $90 billion in 2008 at an average yearly growth rate of 7.2%, according to a report from the Italian Chemical Pharmaceutical Association (CPA).

Also, Astellas and Medivation sign development pact; WuXi PharmaTech appoints VP of business development, more...

The global API market has expanded from $69 billion in 2004 to more than $90 billion in 2008, according to a report from the Italian Chemical Pharmaceutical Association (CPA); in particular, the report noted a rapid growth rate for generic APIs.

Cheaper generic pharmaceuticals, emboldened by US law and patent challenges, restrict novel future innovations.

Company and People Notes: SurModics forms agreement with Roche and Genentech; Hospira names Daphne Jones senior VP and chief information officer; more...