
As new process validation guidelines emerge, industry needs to reinvent how it releases product.

As new process validation guidelines emerge, industry needs to reinvent how it releases product.

The author describes the approach taken to develop a facility dedicated to handling potent and cytotoxic drug substances.
A Project Manager's Perspective.
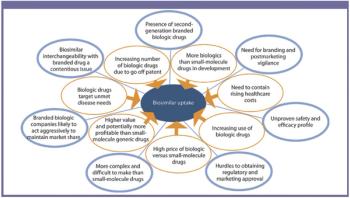
The author reviews the major biopharmaceutical markets' activity and predicts how the markets may evolve.

The authors describe the various available technologies used in orally disintegrating tablets.

Combination products have been researched and administered for many years, some successfully and others not.

Combining drugs with synergistic mechanisms of action yields some indisputable benefits; improved efficacy, reduced dosing, enhanced patient compliance, to name just a few.

Parteck ODT is a newly introduced ready-to-use excipient for fast melt tablets.

The addition of superdisintegrants to oral solid dosage forms can improve disintegration and, in turn, drug dissolution.

The Czech Republic will no longer purchase the H1N1 flu vaccine from Baxter International.

Scientists have modified a tobacco plant to produce a vaccine for norovirus, the viral infection sometimes referred to as the "cruise ship virus".

Scientists have modified a tobacco plant to produce a vaccine for norovirus, the viral infection sometimes called the "cruise ship virus."

Company and People Notes: UCB and Novartis form agreement; AAIPharma appoints VP of regulatory affairs; more...

Researchers at the University of Illinois (US) have developed a cancer drug delivery system that reportedly kills target tumour cells, spares healthy cells and has effects that can be reversed to halt potentially hazardous side effects.

Researchers at the University of Illinois (Champaign) have developed a cancer drug delivery system that reportedly kills target tumor cells, spars healthy cells, and has effects that can be reversed to halt potentially hazardous side effects.

Also, FDA publishes draft guidances of two ICH Annexes; EMEA sets format for compliance advice; more...

Also, XCELERON and GSK form agreement; Millipore appoints VP of life sciences; more...

The US Food and Drug Administration?s Draft Guidance for Industry?Process Validation: General Principles and Practices provides a life-cycle approach for validating pharmaceutical processes and aims to help pharmaceutical companies achieve consistently high product quality.

Cooling water is a critical component in the research and development, bulk manufacturing, and packaging of pharmaceuticals.

Also: DSM's North Carolina facility receives SafeBridge certification; Dynavax CFO to retire; more...

Baxter International (Deerfield, IL), sanofi aventis (Paris), and Novartis (Basel, Switzerland) provided updates last week of their production and regulatory activities relating to preparedness in supplying the A(H1N1) pandemic influenza vaccine. Novartis also outlined its activities for providing seasonal flu vaccines.

The US Food and Drug Administration released its Guidance for Industry titled Pharmaceutical Components at Risk for Melamine Contamination.

Also, Pfizer forms two research agreements in China; NanoInk appoints John Kubricky to its scientific advisory board; more...

Biotech firms must first close the gaps between science and biology on the path toward QbD.
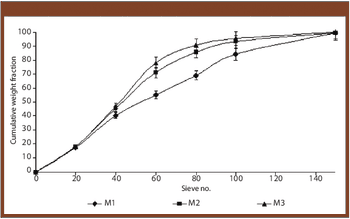
The authors prepared granules containing cinnarizine using polyethylene glycol 6000 as a melting binder and lactose monohydrate as hydrophilic filler. The effects of binder concentration and size were studied.