
Researchers working on extending the shelf life of antibody drugs may find help in a computer model developed by a research team at MIT (Cambridge, MA).

Researchers working on extending the shelf life of antibody drugs may find help in a computer model developed by a research team at MIT (Cambridge, MA).

Also, Isogen completes sterile facility; Dishman appoints head of generic API business; FDA releases final rule on authorized generic drugs and NDA submissions; more...

Two years ago, the US Food and Drug Administration's Office of Generic Drugs (OGD) developed and implemented a program to test question-based reviews for abbreviated new drug applications (ANDAs). OGD is reviewing the benefits and challenges faced by pharmaceutical firms involved in the initiative to see what changes need to be made going forward.

Also, Gilead and Tibotec form agreement; FDA approves 2009-2010 flu vaccine; Catalent appoints VP in packaging unit; more...

Also, Lonza and Medarex sign agreement; Covance appoints VP and chief scientific officer of global analytical services; more...

Also, Lundbeck acquired LifeHealth; FDA is seeking a director of its new tobacco regulation branch; Charles River Labs announces personnel changes; more...

The US Pharmacopeia is revising its monographs for four pharmaceutical excipients: propylene glycol, sorbitol solution, sorbitol sorbitan solution, and noncrystalllizing sorbitol solution.

As the pharmaceutical industry turns its efforts to biologic-based drugs and to more targeted delivery approaches, new tools are needed. Some recent developments are discussed.

Formulators and manufacturers have many options for modifying release profiles in multiple-API products.

Also, Wyeth and Catalyst sign agreement; FDA seeks public opinion about tobacco regulation; Catalent appoints VP of quality and regulatory affairs; more...

Pan coating has been the preferred method of coating tablets for more than 20 years, but core coating is becoming more popular.

The Federal Trade Commission (FTC) last week issued an interim report that examined the effects of authorized generics on competition in the prescription drug market.

The regulators are doing it. But industry's fear of sharing information may leave them behind.
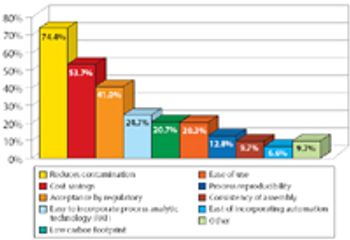
A Pharmaceutical Technology report looks at trends in biopharmaceutical manufacturing. This article contains bonus online-exclusive material.
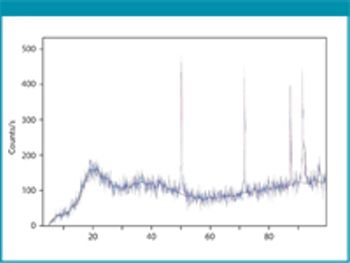
The authors modified gellan gum using microwave technology and showed it can be used as an excipient in tablet formulations.
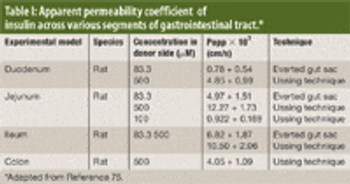
The authors review various oral drug delivery systems that have been explored to increase patient compliance for insulin.

Is it good policy to pay for bad behavior?
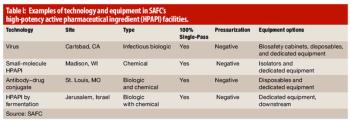
High-potency manufacturing of active pharmaceutical ingredients is a growing and specialized capability.

Traditional Chinese Medicine is widely used, but questions persist regarding its regulatory status.

Developers of low-dose drugs in solid oral dosage forms will find theoretical considerations and practical advice in a new book.
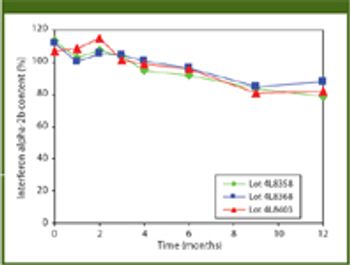
The authors describe a proprietary process for producing a stable, topical interferon alpha-2b formulation that can deliver large drug molecules into the skin or mucosa.
The authors discuss how strategic outsourcing to contract manufacturing organizations that have technical and regulatory expertise can add further value during vaccine development.
Biopharmaceutical companies generally try to avoid paying royalties on novel technologies, including novel expression systems, in part because of the inability to predict revenue flow after a product is commercialized.
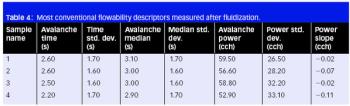
Cefaclor is a β-lactam cephalosporin antibiotic that has a wide particle size distribution. Because of the nonporous nature of the material, the specific surface area value accounts for a significant amount of fine particles possibly present in the samples under analysis.

Also, Adimab forms deals with Merck and Roche; Manhattan Pharmaceuticals' CEO and president steps down; more...