
As the industry continues to evolve, do we know where we're headed?

As the industry continues to evolve, do we know where we're headed?

Laboratory personnel share interesting tales as well as stories of unexpected tails.
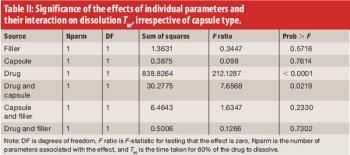
The authors examined the disintegration and dissolution profiles of propranolol and rofecoxib tablets overencapsulated with standard hard-gelatin capsules and with capsules specifically designed for double-blind clinical trials.

As demand for global vaccine development and production grows, all eyes are turning to Asia.

Interphex Showcase 2010.

The BIO convention, and healthcare reform, could re-energize biotech.

Could insect cells offer a faster way of manufacturing pandemic influenza vaccines compared with traditional egg-based methods? According to researchers at the Vienna Institute of BioTechnology (Austria), their new technique could help a virus-like particle (VLP) vaccine to reach the market within 3 months from the first isolation of a new influenza strain - traditionally produced vaccines take approximately 6 months.

Florian Krammer explains how a novel technology using insect cells can accelerate the manufacture of pandemic influenza vaccines.

Pfizer Invests In Nodality; SOCMA Approves New Members; And More.

Merck Ends Partnership With Dynavax; PhRMA Elects Officers; And More.

Abbott Agrees to Acquire Facet Biotech; FDA Issues Black-Box Warning For Plavix; and More.

Three companies joined the University of Pittsburgh Medical Center's 21st Century Biodefense initiative to establish a flexible vaccine development and production facility.
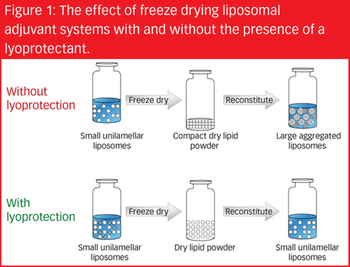
There are a variety of vaccine types, each varying in safety and efficacy, and each possessing its own formulation challenges. To overcome potential instabilities when developing vaccines, one formulation strategy is to produce a dried product.

Exelixis And XenoPort Announce Job Cuts; GSK Dedicates India Facility; And More.

The US Food and Drug Administration recently published guidance for the characterization and qualification of cell substrates, viral seeds, and other biological materials used to manufacture viral vaccines for human use.

Astellas Pharma Makes Bid for OSI Pharmaceuticals; Ringo Retiring From Pfizer; And More.

Could President Obama's tax reform, which is targeted at reducing outsourcing, endanger India's contract-services industry?

Pre-Interphex 2010 Product Releases.
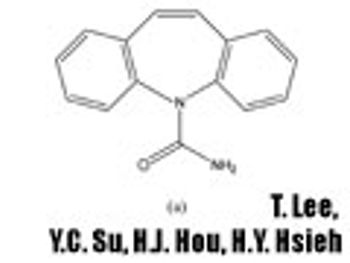
In Part I of this article, the authors describe the materials and methods used in developing a screening strategy to accelerate the preparation and characterization of spherical agglomerates by spherical crystallization.

To best carry out the vision of Hatch-Waxman, Congress must act now on biogenerics.

Brief pharmaceutical news items for March 2010.

Analysis of the opportunities and challenges in the biosimilars market.

From last-minute product inserts to putting out fires, close calls are a common occurrence.

If both sides of the aisle don't agree on even mild healthcare reform soon, the bill could die out.

Although efforts have been made to improve solubility, many poorly soluble drugs still reach the market. Peter Nielsen discusses new developments in solubilisation technology, which could help those companies seeking to reinvigorate marketed products.