
Also, Sandoz received approval for its third biosimilar from the EU, WuXi PharmaTech's CFO Benson Tsang to leave at month's end; more...

Also, Sandoz received approval for its third biosimilar from the EU, WuXi PharmaTech's CFO Benson Tsang to leave at month's end; more...

The US Food and Drug Administration withdrew this week its direct final rule on Reporting Information About Authorized Generic Drugs.

Also, PPD to acquire AbC.R.O.; Bilcare Global Clinical Supplies named Tony Moult general manager of Bilcare GCS Europe; more...

Also, recalls for two KV Pharmaceutical subsidiaries; Human Genome Sciences delivers anthrax drug to US Strategic National Stockpile; Akorn president and CEO leaves the company; more...

The Federal Trade Commission has filed a complaint in federal district court challenging agreements in which Solvay Pharmaceuticals (Marietta, GA) paid generic drug makers Watson Pharmaceuticals (Corona, CA) and Par Pharmaceutical Companies (Woodcliff Lake, NJ) to delay generic competition to Solvay's branded testosterone-replacement drug "AndroGel," a prescription pharmaceutical with annual sales of more than $400 million, according to an FTC press release.

The authors formulated and developed taste-masked RDFs of cetirizine hydrochloride for patients who experience difficulty in swallowing the tablet dosage form of the drug.

A recent reader poll focused on predictions for the industry's future.

FDA's role should not be overlooked as it has been in years past.
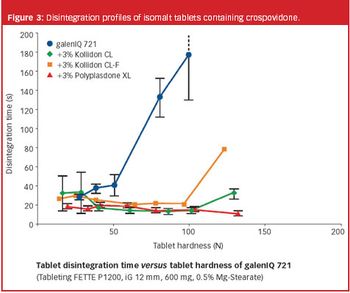
During the past years, there has been increasing demand for fast dissolving disintegrating tablets (FDDTs), such as orally disintegrating tablets (ODTs) and sublinguals.

A discussion of the basic principles of immunity and the nature of antibodies.

Also, UCB will divest certain business in emerging markets to GSK; the KineMatik Group named Michael G. Jarjour president and CEO; more...

Also, Elan explores strategic alternatives; NanoGuardian appoints John D. Glover to lead its Security Advisory Board; more...

The US Department of Health and Human Services's Biomedical Advanced Research and Development Authority has awarded Novartis a contract valued up to $486 million over eight years to support the design, construction, validation, and licensing of a US cell-based influenza vaccine manufacturing facility in Holly Springs, North Carolina.

One relatively new dosage form is the orally dissolving strip, a thin film formulated with hydrophilic polymers that rapidly dissolves on the tongue.

Also, Bristol-Myers Squibb forms collaboration with ZymoGenetics, Hana Biosciences appoints VP of regulatory affairs, more...

Also, Novozymes Biologicals settles pollution case with the US Department of Justice; EntreMed restructures management team; more...

Outsourcing sterile manufacturing involves an integrated approach in product life-cycle management.
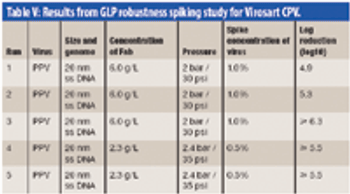
The authors examine the challenges of integrating a large-scale chromatography and nanofiltration process for purification of a polyclonal antibody.

The incoming administration has renewed hope for stem cells, but less adequate copycats may follow.

A book explains the many pharmaceutical applications of polymers from natural sources.

Brief pharmaceutical news items for January 2009.

Instead of hampering progress, cost pressures might inspire Big Pharma to get creative.

The biotechnology sector is occasionally described as a rainbow, with each sub sector having its own colour. But what do the different colours of biotechnology have to offer the pharmaceutical industry?
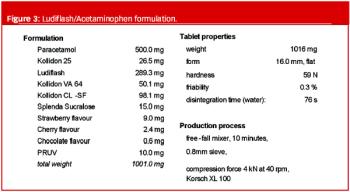
A new excipient for orally disintegrating tablets not only imparts superior tablet characteristics, but has the added advantage of allowing users to maintain full control over their formulations, manufacturing processes and intellectual property.

A new year, all things being equal, is a time of looking to the future with positive intent and traditional bonhomie. At the very least, it suggests new beginnings. As we enter 2009, however, the world is moving into a major recession and things are far from equal.