
BioOutsource releases informational video detailing issues associated with ADCC assays and how to effectively analyze them.

BioOutsource releases informational video detailing issues associated with ADCC assays and how to effectively analyze them.

The UK’s National Biologics Manufacturing Centre will use Novasep’s BioSC Lab for protein purification.

Almac is investing GBP16 million to expand its formulation and analytical development services and has successfully negotiated to operate in the Charnwood Campus in Loughborough, England.

Even though rising production and use of generic pharmaceuticals is saving billions for the nation’s healthcare system, policy makers continue to slap the industry with policies it claims will limit product development and sales.

GE Healthcare's KUBio prebuilt modules were shipped from Germany to JHL Biotech in Wuhan, China.

Biotech boom, niche markets, smaller batch sizes and high potency manufacturing are among the key trends shaping the pharmaceutical industry of the 21st century, according to Christian Treitel from Bosch Packaging Technology.

South Africa’s Biovac Institute, which develops and produces vaccines for the country, launched a public-private partnership with Pfizer to enable local manufacturing of Prevenar 13, a vaccine against pneumonia-causing bacteria.

At least 70 patents for Humira will protect the legacy product from biosimilar competitors, according to information presented during the company’s third-quarter earnings call.
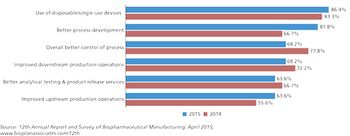
Better process development is creating industry benchmarks for bioprocessing.

The new Syrina 2.25 is a compact auto-injector that uses the standard 2.25 mL prefilled syringe, based on Bespak’s proprietary VapourSoft technology.

The Transform hydrate-able film from Lubrizol LifeSciences is compatible with a range of APIs.

The determination of topical bioequivalence requires a multi-faceted approach, tailored specifically to the generic-drug formulation.

Sartorius Stedim Biotech’s Flexsafe 3D Pre-designed Solutions meet ASTM D4169 Standard Practice requirements and are designed for storage and shipping of biopharmaceutical fluids.

ProBioGen plans to develop and scale-up production of a biosimilar to the mAb cancer drug trastuzumab and will transfer the commercial manufacturing process to Indonesia’s government-owned Bio Farma.

USP responds to FDA's draft guidance on the naming of biological products.

Catalent’s SMARTag™ technology enables biologic innovators to develop more efficacious antibody drug conjugates. It allows site-specific, programmable drug placement using proprietary cytotoxin-linkers/conjugation chemistry in an efficient and scalable process.

Capsugel has launched its enTRinsic drug-delivery technology platform. Using pharmaceutically approved enteric polymers, the technology offers full enteric protection without the need for functional coatings and enables targeted release of gastric acid- and heat-sensitive active ingredients in the upper gastrointestinal tract. The new technology platform expands Capsugel’s range of modified- and targeted-release solutions and biotherapeutic formulation offering, and can be used for oral delivery of drugs, including vaccines, proteins, and peptides.

According to a third-quarter earnings report, Pfizer’s vaccine business contributed nearly 14% to its total revenue.

Despite considerable investment by biotech manufacturers in developing competitive biologics for the US market, gaining FDA approval of these products has turned out to be a slow and complex process.

EMD Millipore, the life-science division of Merck KGaA, has introduced Parteck SRP 80, a new functional excipient for oral sustained-release formulations. The excipient is polyvinyl alcohol (PVA)-based and fully synthetic-according to EMD Millipore, this feature ensures batch-to-batch and performance consistency and facilitates quality by design (QbD) and validation processes.

Univercells will integrate its single-use bioprocess with the Takeda vaccines production platform to allow local production.

Biovest’s mAbVault provides supply-chain redundancy for antibodies that enables customers to lock in price-per-gram protein production.

Hovione is investing in specialized formulation capabilities, beginning with the acquisition of a formulation facility adjacent to the current process chemistry and particle engineering facility in Loures, Portugal, the company announced on Oct. 19, 2015. This acquisition is a strategic investment to further boost development and manufacturing capabilities for both inhalation and oral dosage forms.

Scottish injectable-drug manufacturer Symbiosis Pharmaceutical Services plans to expand its sterile filling facility.

Steris Corporation’s steam sterilizer provides reliable decontamination for high-containment facilities.