
Matthieu Egloff, product director with ATMI LifeSciences, discusses the need for larger, special reactors that can provide the necessary conditions for the production of fragile cells on a larger scale.

Matthieu Egloff, product director with ATMI LifeSciences, discusses the need for larger, special reactors that can provide the necessary conditions for the production of fragile cells on a larger scale.

Roche and BioLamina have entered into a research and development agreement to jointly develop new cell culture systems for various applications, including stem cell research. The collaboration will assess laminin-based in-vitro cell culture matrices that can offer physiological microenvironments for living cells.

Mylan announced that it has signed an agreement to acquire the generic injectables unit Agila Specialties from Strides Arcolab for $1.6 billion in cash.

Companies risk drowning in alphabet soup if the latest three-letter acronym improvement strategy isn't clearly linked to business strategy.
Engineering nanoparticles with optimal properties for use in cancer therapies.

Visual mapping can provide a particle-size distribution estimate.

Vaccine development is benefiting from manufacturing advances and support for global health.
Pharmaceutical Technology brought together a panel of industry experts for a special forum to discuss solubilizing polymers and the related formulation strategies for poorly soluble drugs.

Capsugel’s acquisition of Encap Drug Delivery expands its lipid expertise and adds an FDA-inspected commercial manufacturing facility.

The tableting science anti-research (TSAR) project seeks to understand why certain formulations stick to tablet tooling.
A roundtable discussion of the challenges and innovations in tablet splitting featuring Freeman Technology, Accu-Break Pharmaceuticals, and Medelpharm.

An alternative chapter has been added to the European Pharmacopoeia for dosage uniformity.

Custom manufacturers with expertise in highly potent API production discuss the latest issues and technology developments in the field.

Kevin Ott, executive director of the Bio-Process Systems Alliance, discusses the latest trends and issues surrounding single-use technologies and the role of his association.

Belgium drug developer Ablynx and UK-based Spirogen have entered into a research collaboration to discover and develop novel anticancer drug conjugates combining Spirogen's proprietary cytotoxic drugs, pyrrolobenzodiazepines (PBDs), and the company's associated linker technology, with nanobodies generated using Ablynx's proprietary technology platform.

The EMA's Committee for Medicinal Products for Human Use (CHMP) has recommended Sanofi's six-in-one pediatric vaccine for marketing authorization.

FDA approved Roche?s antibody-drug conjugate, Kadcyla (trastuzumab emtansine or T-DM1), for the treatment of people with HER2-positive metastatic breast cancer.

A GBI Research report says the opioid market will be driven by development of new products and post-marketing studies designed to reduce abuse risk.

Merck and Samsung Bioepis have formed an agreement to develop and commercialize multiple prespecified and undisclosed biosimilar candidates.

Protein Sciences announced that the Biomedical Advanced Research and Development Authority (BARDA) will continue to support the company?s influenza vaccines program.

Heated magnetic nanoparticles consisting of a liposome nanocontainer with superparamagnetic iron oxide nanoparticles are among the recent advances in nano-based drug delivery.
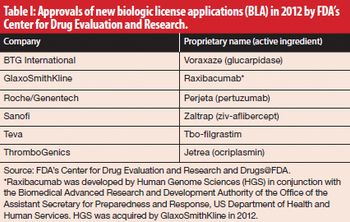
The share of biologic-based drugs in the global pharmaceutical market is on the rise.

GlaxoSmithKline has formed a joint venture with Indian vaccines company Biological E to research and develop a six-in-one combination pediatric vaccine to help protect children in India and other developing countries from certain infectious diseases.

FDA has approved Protein Sciences's FluBlok, a seasonal influenza vaccine made with novel technology. FluBlok uses recombinant DNA and a modified baculovirus (a virus that infects insects) to produce a safe and effective human flu vaccine. FDA approved Flublok for people 18–49 years old.

The PATH Malaria Vaccine Initiative and Inovio Pharmaceuticals have announced a follow-on collaboration to advance the development of malaria vaccines and new vaccine delivery technologies.