
Following passage in the California Assembly, the California State Senate passes legislation to specify requirements for dispensing biosimilars.

Following passage in the California Assembly, the California State Senate passes legislation to specify requirements for dispensing biosimilars.

FDA approves BD Simplist product ondansetron injection, an injectable antiemetic.

Merck & Co gets FDA approval to manufacture bulk varicella at its in Durham, North Carolina for use in chickenpox and shingles vaccines.

Baxter and Coherus Biosciences colaborate to develop and commercialize a biosimilar version of etanercept for Europe, Canada, and, Brazil.
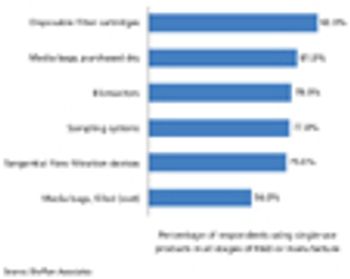
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Prequalification of Sanofi Pasteur?s Menomune vaccine makes it eligible for purchase by United Nations agencies.

Avacta?s Optim 2 instrument provides a practical solution for determining the conformational stability of adjuvant bound vaccines, eliminating the need for awkward and time consuming standard analytical techniques. [more?..]

The new subcutaneous formulation of Herceptin can be administered six times faster than the standard intravenous formulation.

The advantages of using an automated powder dispensing system in a ventilated balance enclosure for efficient handling and effective containment of potent compounds are discussed.

Industry experts discuss the requirements and challenges involved in getting a biosimilar product from bench to launch.
The authors describe the background and applications of fully formulated aqueous shellac for film-coating systems.

A Sanofi Pasteur flu-vaccine trial in adults 65 years of age and older meets a primary endpoint for superior efficacy.

Sanofi Pasteur has purchased Freeslate?s Biologics Formulation System for accelerating research in vaccine and protein formulation development.

FDA's draft guidance provides answers to questions received on FDA's abbreviated new drug application stability guidance.

Conventional cell-imaging systems that provide high quality data can be expensive and complex to use. New systems from Biotek Instruments are designed to overcome these issues.

Installation of a quaternary chiral center with high enantioselectivity using memory of chirality enabled the six-step synthesis of the desired active compound.

Extensive adoption of single-use technologies and unidirectional flow reduces cross-contamination risk in the company?s new biomanufacturing facility.

Conventional cell-imaging systems that provide high quality data can be very expensive and complex to use. New systems from Biotek Instruments are designed to overcome these issues.

Reversible nanoencapsulation technology may make it possible to deliver high molecular weight drugs directly to target cancer cells, increasing efficacy and reducing side effects.

Rapid screening of critical API properties can quickly identify the best approach for increasing bioavailability.

The companies initially focused on five biosimilar products.

Adamis Pharmaceuticals agrees to license and potentially acquire 3M's Taper Dry Powder Inhaler technology.

Pfizer, Merck, Sanofi, and AstraZeneca are among the companies reporting revenue declines from generic-drug incursion. A look at what the companies are doing to stimulate growth.

A new study reveals how a promising anticancer specifically kills prostate cancer cells by compromising their ability to withstand environmental stress.
Extensive comparability testing is required to ensure that biosimilars have comparable profiles to their reference products.