
The divide between innovation and conflict of interest in medical research is not so clear.

The divide between innovation and conflict of interest in medical research is not so clear.

Genotoxic impurities and how to identify them and control for them have been a concern for several years in the pharmaceutical manufacturing industry. Pharmaceutical Technology spoke with Bo Shen, PhD, principal scientist at Amgen and chair of the AAPS Pharmaceutical Trace Impurities Focus Group, to gain insight on key challenges.

In Part II of a three-part article, the authors examine impurities from chiral molecules, polymorphic contaminants, and genotoxic impurities.

Wirelessly controlled microchips may offer an alternative to injection-based drug delivery

SOCMA's Bulk Pharmaceuticals Task Force outlines key goals and challenges for user-fee legislation.

Has the long-awaited guidance answered all of the industry's questions?

The author reviews significant changes to GMP for excipients in the forthcoming American National Standard, including a risk-based approach to excipient manufacture, why new requirements were proposed, and their potential impact to excipient manufacturers.

This article provides guidance for industry on how to comply with the pending American National Standard on excipient GMP, with a focus on risk assessment.
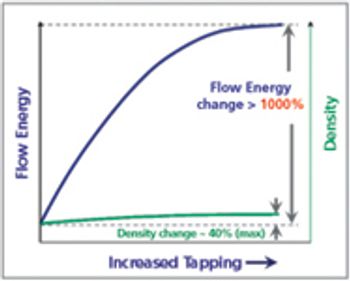
This article considers the different conditions to which the powder is subjected in the tableting process, and discusses which powder properties should be measured to accurately reflect likely powder behavior in the process.
Pharmaceutical companies, equipment providers, contract-service providers, and excipient manufacturers apply various approaches for improving solubility. The article examines some recent developments.

Taste-masking is an important consideration to ensure patient compliance.

Experts in solid dosage discuss the formulation and manufacture of multilayer tablets.

The pharma industry has reached the long-dreaded patent cliff, but for copycat products, business is booming.

Boehringer Ingelheim's Heribert Häusler tells us about parametric release and real-time testing.

JHS Secures Four Sterile Parenteral Products Manufacturing Contracts; Samsung Biologics, Biogen Idec Establish Biosimilars Joint Venture; and More.

Pfizer has signed an agreement with the Chinese biopharmaceutical company Zhejiang Hisun Pharmaceuticals with the objective of establishing a $545-million joint venture to develop and commercialize branded generic medicines in both China and the global market.
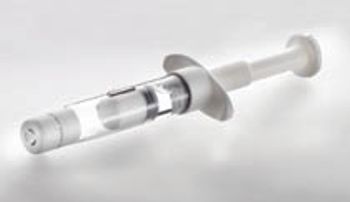
The author discusses the relative advantages and disadvantages of lyophilization in vials and dual-chamber systems.

A nickel's worth of free advice to the competition could come at the expense of your bottom line.

A new class of nanoparticles hold promise for preventing premature drug release and offering greater accuracy and effectiveness in drug delivery.

New product reviews for February 2012.

Debottlenecking downstream mAb purification.

Experts discuss solutions for filter bacterial retention and related challenges. Contains online bonus material.

Does nanotechnology offer a cure-all or a kill all? We speak with Ruth Duncan about the real potential of nanomedicines.

We bring industry experts together to discuss the importance of self-administration and what injection technologies are best suited to this cause.

The generic-drugs market is poised to experience strong growth as key blockbuster products go off patent, but companies looking to benefit from this will have to be careful about the product segments where they compete, according to a report from Frost & Sullivan.