
Partnerships between industry and academic medical centers are expanding to meet R&D needs.

Partnerships between industry and academic medical centers are expanding to meet R&D needs.

Does global development have to entail multiple comparability studies?

A number of factors need to be considered when evaluating preclinical dose-formulation stability. The authors discuss formulation, storage and dosing conditions.

Coherus BioSciences, Daiichi Sankyo Form Biosimilar Pact; Sandoz Agrees to Acquire Fougera Pharmaceuticals; and More.

AstraZeneca's CEO David Brennan To Retire; PRA, Amgen Reach New Biosimilar Agreement; and More.

The Obama administration details five strategic imperatives to drive bioscience research as a means of economic growth.

Targeted polymeric nanoparticles are an important vehicle for controlling and targeting dosing of chemotherapeutic agents.

Industry experts working with extended-release injectables discuss challenges and solutions to formulating and manufacturing these complex products.
The author discusses potential opportunities to improve the patient experience through formulation and delivery device technologies.
In this technical forum, experts describe different methods of rapid microbial testing and their applications.

If a product does not have its own antimicrobial properties, then a preservative must be used to ensure microbiological safety.

The European Commission is offering a EUR 2-million ($2.64 million) inducement prize to encourage innovation in the area of cold-chain logistics and vaccine stability.

The growing use of high-potency APIs is leading to changes in how the pharmaceutical industry looks at containment.

Ranbaxy Laboratories announced that beginning in March 2012, the first shipments of atorvastatin, the generic version of Pfizer's Lipitor, had been sent to US markets from its new Mohali manufacturing facility located in Punjab, India.

On Mar. 29, 2012, the Generic Pharmaceutical Association reiterated its call for Congress to move forward with user-fee proposals for generic drugs and biosimilar products.
In Part III of a three-part article, the authors examine various degradation routes of APIs, impurities arising from interactions in formulations, metabolite impurities, various analytical methods to measure impuritie, and ways to control impurities.

Critical issues that should be considered when scaling up a hot-melt extrusion process.

With financing constrained, biotechnology firms must find ways to sustain innovation.

How to use geographic diversification and legacy technology transfers to avoid product shortages.

Excipient manufacturers expand production capacity and partner to broaden their offerings.
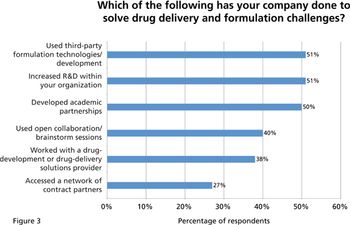
A recent survey examines the industry's views on the chief challenges and technologies in drug delivery and formulation development.

Charles E. Seeney tells us about the possibilities of nanotechnology and magnetism, and how a novel approach could improve localised drug delivery.

In our tablet coating process, we are losing up to 15% of our coating solution in each processing run. What can we do to prevent this problem in order to reduce waste and increase our cost efficiency?

On Mar. 7, 2012, GE Healthcare announced an agreement to acquire Xcellerex, a supplier of manufacturing technologies for the biopharmaceutical industry, for an undisclosed amount.

AstraZeneca Files Suit Against FDA; Pfizer, Biocon Terminate Biosimilar Alliance; and More.