
The reopened debate over embryonic-stem-cell research could stifle many other scientific pursuits.

The reopened debate over embryonic-stem-cell research could stifle many other scientific pursuits.
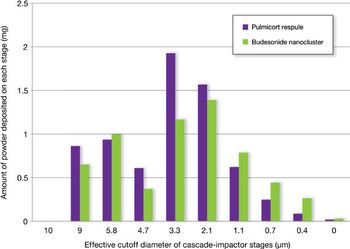
The authors describe a technique designed to yield low-density powders with a tailored particle-size distribution over a broad range of respiratory flow rates.

A recently released industry guide outlines a science- and risk-based approach to control the risk of cross-contamination.
The growth of biologics is an important factor for the injectable-drug delivery systems market. A look at the technical and market considerations affecting this sector.

Private companies and universities are developing new ways to deliver protein drugs.
Drugmakers hatch new manufacturing paradigms in the wake of the 2009 H1N1 influenza pandemic.

From disagreement to denial, being cordial about quality control can be challenging.

A look at MVI's malaria work in developing countries.

Novartis' Matthew Stober discusses vaccine manufacturing, including egg- and cell-based systems.

In light of compendial changes, representatives of the US Pharmacopeia and an industry consortium provide perspectives on cap and ferrule labels.
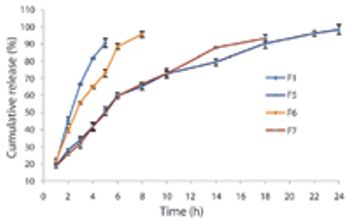
The authors developed a formulation for effervescent gastroretentive drug delivery techniques using ibuprofen as a model drug. They optimized the formulations by applying full factorial design.

President Obama and HHS eye innovation and countermeasures to protect public health.

The early part of the decade saw a decline in vaccine sales and manufacture, but finally the industry is bouncing back, with Europe particularly well placed to make an impact.
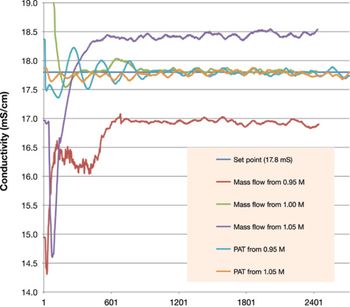
The authors describe the operational requirements and design of a process-ready PAT-based IBD system.

The author describes the development of small-angle X-ray scattering and analyzes its advantages in the characterization of drug-delivery systems and large molecules. This article is part of a special Analytical Technology issue.
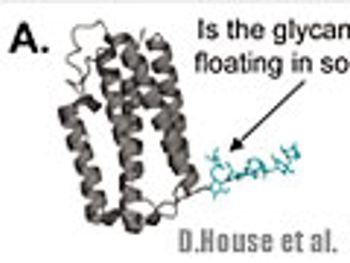
The authors review and discuss the influence of glycans on the conformation of a representative IgG1 biopharmceutical using H/DX-MS as an analytical tool.

Novartis Sells US Enablex Rights; Ricerca Names Chemistry Director; And More.

DSM and PolyTherics in Development Deal; Kite Pharma Appoints President and CEO, And More.

Genzyme Sells Genetics Business; Bausch and Lomb Names Vice-President; and More.

The generic-drug company Actavis (Hafnarfjordur, Iceland) is considering acquiring a 51% stake in the biopharmaceutical company BioPartners Holdings (Barr, Switzerland) from Bioton, a Polish biotechnology company.
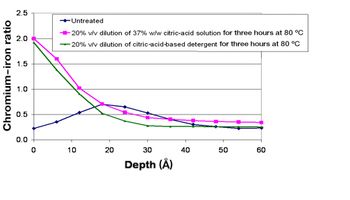
The author describes the types and sources of rouge and explains ways to prevent and mitigate this problem.

US Pharmacopeia apparatuses for testing the dissolution of transdermal drugs produce good, reproducible results. Yet some scientists believe that further modifications could improve the instruments? suitability for this application.

Director General of the European Generic Medicines Association (EGA), Greg Perry, has emphasised the need for EU biosimilar guidelines for monoclonal antibodies (mAbs), as well as a harmonised, global approach to biosimilars in general, at the agency's 8th International Symposium on Biosimilar Medicines.

Pfizer to Acquire FoldRx; Protalix Appoints COO; And More.

Director General of the European Generic Medicines Association (EGA), Greg Perry, has emphasized the need for EU biosimilar guidelines for monoclonal antibodies (mAbs), as well as a harmonized, global approach to biosimilars in general, at the agency's 8th International Symposium on Biosimilar Medicines.