Editors' picks of pharmaceutical science and technology innovations.

Editors' picks of pharmaceutical science and technology innovations.

Abuse-deterrent combination drugs represent a niche area in formulation development.

USP is working to ensure quality standards and to increase public information.

An analysis of the approaches and tools used to tackle the problem of poorly soluble drugs.

Today begins the two-day public hearing being held at FDA headquarters in Rockville, MD, to gain public in put on how to implement and regulate the follow-on biologics pathway that was cleared via President Obama's Biologics Price Competition and Innovation Act of 2009, which falls under the 2010 approved healthcare reform legislation.

As technology advances, industry's needs are growing.

Scientists and practitioners must work together for the overall good of the patient.
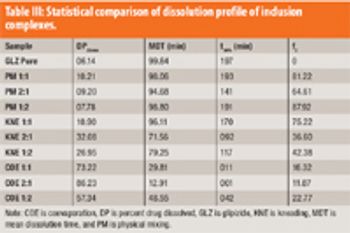
The authors describe the development of an inclusion complex of GLZ and formulated an extended-release dosage form based on osmotic technology.

Remaining calm, cool, and collected during mergers and inspections is a feat in itself.
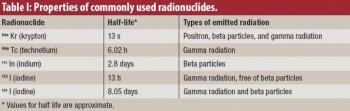
The authors discuss gamma scintigraphy as a technique for in vivo evaluation of drugs and delivery systems.

The authors demonstrated that ODTs can be obtained by direct compression of a mixture of starch, fructose, and SMCC.

Connecting science and policy might increase support for innovation.

The authors present an update to the Wyeth/BASF experience with the IPEC Novel Excipient Safety Evaluation Procedure.

Stuart Needleman, president of active-ingredient development and manufacturing at Aptuit, discusses industry trends and challenges.

The author reviews key considerations for formulating powders for use in inhalers. This article is part of a special Drug Delivery issue.

Microbubbles can temporarily open many biological barriers for polar molecules, macromolecules, and particles. Scientists have brought well-known contrast agents back to the laboratory and redesigned them as drug carriers. This article is part of a special Drug Delivery issue.

A review on the current status of long-acting injectables, including commercially marketed products. This article is part of a special Drug Delivery issue.

The authors describe an alternative approach to compressing and coating minitablets for use in a sustained-release, solid oral-dosage form. This article is part of a special Drug Delivery issue.

The author outlines how to choose carriers and capsule shells according to dosage requirements and intended use. This article is part of a special Drug Delivery issue.

The authors provde a review of melt extrusion's evolution and applications in the pharmaceutical industry. This article is part of a special Drug Delivery issue.
The author reviews advances in technology that may soon allow transdermal delivery of two of the fastest growing drug classes on the pharmaceutical market. This article is part of a special Drug Delivery issue.

Although there are no regulatory requirements or established pharmacopoeial techniques for the dissolution testing of inhaled drugs, such testing can potentially open up the opportunity to tailor formulation properties.

PPD and Bend Research Form Collaboration; Ricerca Makes Senior Appointment; And More.

Pfizer Forms Pact with Biocon; Baxter Makes Appointments; And More.

The European Generic medicines Association (EGA) has made a number of recommendations on how to ensure the quality of medicines in the globalized supply chain; in particular, the EGA spoke about API quality and quality supervision, and how these can be improved by transparent communication between authorities and industry.