
O-arylation and O-alkylation, a one-pot protein synthesis, a combined approach in continued and chemocatalysis, and green-chemistry applications are the target of some recent advances in API synthesis.

O-arylation and O-alkylation, a one-pot protein synthesis, a combined approach in continued and chemocatalysis, and green-chemistry applications are the target of some recent advances in API synthesis.

New studies reveal the promise and feasibility of transdermal vaccine delivery.

Terry Novak, president of Norwich Pharmaceuticals, on recent industry trends.

A path to personalized medicines creates a new paradigm for development and manufacturing.
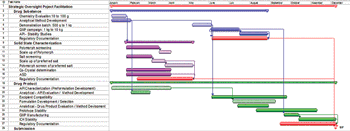
The article examines a cross-functional supplier integration model to facilitate project management.

Pfizer's Experience with QbD. This article is part of a special issue on Outsourcing.
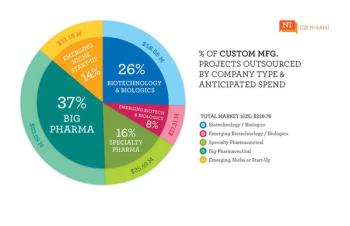
The article examines the drivers of customer perception of contract service providers of pharmaceutical development and manufacturing.

An Industry Roundtable Moderated by Patricia Van Arnum and Rich Whitworh. Contract service and technology providers share their perspectives on the influence of quality by design in the expectations between sponsor companies and outsourcing providers.

FDA Approves the Influenza Vaccine Formulation for the 2011-2012 Flu Season.

A bill introduced by Senators Scott Brown (R-MA), Ron Wyden (D-OR), and John McCain (R-AZ) on July 13, 2011, aims to encourage states to reduce Medicaid spending by offering financial incentives to substitute generic drugs for branded ones where possible.

Even though manufacturers are responsible for ensuring the quality of combination products, some companies may not be certain about what quality system to apply to their production.

FDA, in cooperation with IPEC, is building a spectral library of excipients to detect improper ingredients within a drug product on site.

The performance of biotechnology venture capital and investment is lackluster at best.

A new report places pharmaceutical and healthcare companies ahead in corporate and social governance.

Follow-on versions of complex biologics require extensive expertise in development and regulatory procedures.

High demand could lead to innovation in controlled-release injectables.

Pharmaceutical manufacturers look to various solutions to resolve the challenge of poorly soluble drugs

The authors investigate the influence of hydro-alcoholic media on hydration and drug release from polyethylene oxide extended-release matrices.
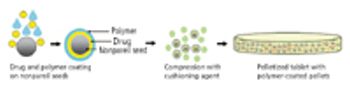
The current review describes the role and selection of excipients, pellet core, coating materials, and compression with various cushioning agents.

A technical leader in solid dosage forms Pfizer CentreSource provides analytical, technical, regulatory, developmental, manufacturing and packaging support that spans the specialized realm of highly potent solid oral dose drug products.

Merck and Hanwha Chemical have formed an exclusive global agreement to develop and a commercialize a biosimilar of Enbrel, a drug to treat moderate to severe plaque psoriasis, psoriatic arthritis, and moderate to severe rheumatoid arthritis.

Effective containment in API and drug-product manufacturing encompasses a variety of process, equipment, and operational issues.

Can microdosing make medicines safer and more effective for children?
More sophisticated biological expression systems expand the functionality of the traditional systems for protein synthesis.

Academic–industry partnerships are increasingly important in biopharmaceutical innovation.