
When it comes to immediate-release tablet formulations, the choice of disintegrant can have a significant effect on the rate and extent of drug dissolution.

When it comes to immediate-release tablet formulations, the choice of disintegrant can have a significant effect on the rate and extent of drug dissolution.

Teva Pharmaceutical Industries agreed to pay shareholders $460 million in cash to acquire a 57% stake in Taiyo Pharmaceutical Industry. Teva also will offer to buy all outstanding shares of Taiyo.

FDA is asking for input on the development of a user-fee program for biosimilar and interchangeable biological product applications.

Alkermes purchased Elan?s Elan Drug Technologies (EDT) unit in a cash and stock transaction worth approximately $960 million. The two companies will be merged into a new holding company in Ireland under the name Alkermes.

PhRMA Urges Congress to Reauthorize Legislation for Pediatric Drugs.

Emerging methods could provide alternative ways of producing inhalable drug particles.

Nanosponges, a controlled-release nanoparticle system, shows promise in targeted drug delivery

Can the semiconductor industry help Big Pharma develop therapies?

Innovator and generic-drug companies need to adapt to compete in the biosimilars market.

The author reviews the state of downstream processing and considers potential solutions, including the streamlining of full processes and borrowed technologies.
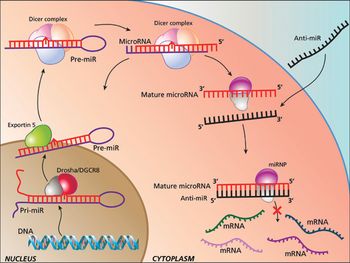
The authors provide further insight into microRNA biology, and the simplicity of anti-miR oligonucleotide drug delivery.

The author describes recent developments to help overcome the downstream processing bottleneck. This article is part of a special issue on Sterile Manufacturing and Bioprocessing.

Linking peptides to polyethylene glycol, or PEGylation, has helped improve pharmaceutical therapeutics in several ways. A wave of new techniques is now ushering in further advances.

The IMS institute has released its report on the use of medicines in the United States during 2010.

Axcan acquires Mpex Pharmaceuticals; Bend Research receives patent for improving bioavailability of low-solubility drugs; and More.

On Tuesday, Apr. 19, 2011, officials from FDA, the US Department of Health and Human Services, and the Drug Enforcement Administration introduced President Obama?s plan for curbing prescription-drug diversion and abuse.

FDA recently published guidance for preventing the cross-contamination of finished pharmaceuticals and active pharmaceutical ingredients with nonpenicillin beta-lactam antibiotics.

Increased competition, the rising role of emerging markets, and targeted opportunities in niche segments are factors influencing the generic-drug market and related supply base.

An uncertain regulatory environment affects funding for biotechnology.

This technical forum is part of a special issue on Solid Dosage and Excipients.

The main challenge for tablet manufacturers processing highly potent APIs (HPAPIs) is to protect equipment operators from the inhalation of airborne particles and prevent skin contact with the product during the entire production process: dispensing, granulation, tablet compression, coating and packaging.
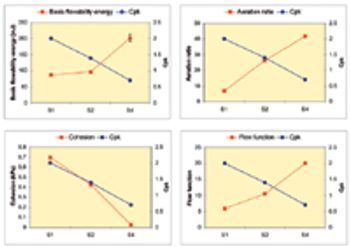
The author explains how to gain an understanding of the relationships between powder characteristics and process performance to match filling-machine geometry to the demands of specific formulations.

The author focuses on how industry can build a system for Total Excipient Control.
Representatives from Pfizer R&D, DEM Solutions, Colorcon, and ARmark Authentication Technologies provide insight into recent tablet-coating technologies.
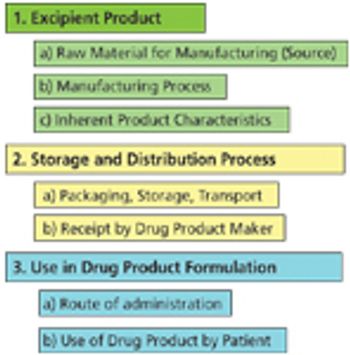
The author describes key considertions for a complete risk-assessment model and provides insight into a pending IPEC guideline in this area.