
The US Food and Drug Administration issued a final guidance last week regarding investigational new drug applications for human gene therapy.

The US Food and Drug Administration issued a final guidance last week regarding investigational new drug applications for human gene therapy.

Dave Drew, group pharmaceutical director of Matcon (Moreton-in-Marsh, UK), says that lean production is essential for drugmakers to survive in the current environment.

Takeda Pharmaceutical agreed to acquire Millennium Pharmaceuticals for $8.8 billion in cash or $25 per share.

Also, Alcon plans to open Singapore facility, Pharmacopeia president and CEO retired, more...

Also, Jubilant Organosys to acquire DRAXIS Health, PPD's Paul Covington to retire, more...

Schering-Plough announced a major new productivity transformation program with a goal of achieving $1.5 billion in annual savings.

US pharmaceutical and biotechnology research companies invested $58.8 billion in research and development in 2007, according to analyses by the Pharmaceutical Research and Manufacturers of America and Burrill & Company.

Also, VaxGen and Raven terminate merger agreement, Darren Head appointed CEO of Cytovance, more...

The economic case for personalized medicine offers a solution to the industry's recent woes.

A changing regulatory environment is on the horizon for excipient suppliers and users.
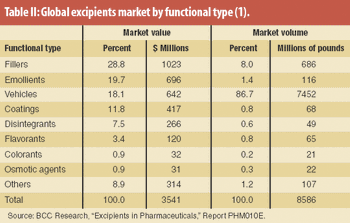
Moderate growth is projected for the global excipients market. Excipient producers target blends and new grades for improving functionality and performance.

Financial experts share their insights for the performance for innovator drug companies and generic-drug players.

The FDA itself issues a cry for help. Is anybody listening?

Excipient producers and industry observers share their perspectives on innovation for excipients.

Heparin contamination casts a shadow on regulatory oversight of product quality.

Brief pharmaceutical news items for April 2008.
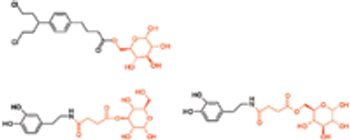
The blood–brain barrier (BBB) forms an interface between the circulating blood and the brain, and functions as a tremendously effective barrier for the delivery of potential neurotherapeutics into the brain parenchyma. Conversely, the BBB possesses various carrier-mediated transport systems for the uptake of small molecules, such as essential nutrients and vitamins. These transporters have become an attractive target for drug/prodrug design in an attempt to ferry drug molecules across the BBB. Central nervous system (CNS) drug delivery is often limited by poor brain penetration of the potential drug candidate. As a result of its unique barrier properties, the BBB poses a huge challenge for the delivery of potential neurotherapeutics into the brain parenchyma.1 It is estimated that only 2% of small-molecule drugs and ,0.1% of novel protein and peptide pharmaceuticals developed for CNS diseases reach therapeutic concentrations in the brain.2,3 Many of the pharmacologically active drugs tend to fail..
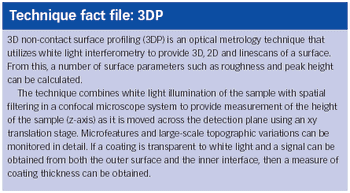
Our company is involved in developing and manufacturing APIs that can be utilized with drug-eluting stents (DES). Despite ensuring constancy in pharmaceutical composition, we are experiencing issues with variations in drug release during in vitro studies. We are working closely with a stent manufacturer to develop the system, but could surface analysis techniques investigate the problem further?
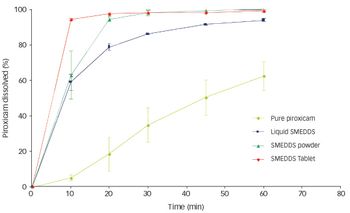
Spraying techniques can be used to produce powder form formulations. The concept works by the adsorption/absorption of a liquid SELF onto a neutral carrier…

Also, Alkermes announces restructuring and reduction of workforce, Icagen announces several senior management promotions, more...

The US market for prescription pharmaceutical grow only 3.8% in 2007, the lowest growth rate in more than 40 years, according to a recent analysis by IMS Health.

Also, Pipex Pharmaceuticals implements cost-cutting measures, Pfizer's Senior Vice-President and General Counsel Allen Waxman leaves the company, more...

The US House of Representatives Committee on Homeland Security approved legislation last week that mandates inherently safer technologies (IST) as part of chemical-site security standards, a move that was opposed by the Synthetic Organic Chemical Manufacturers Association.

Also, PDL BioPharma will no longer pursue sale of the company, executives resign from Topigen Pharmaceuticals, more...

Also, Millipore plans to open Singapore facility, Michael J. Simms joins Alexza Pharmaceuticals, more...