
Merck & Co. and Pfizer signed separate pacts for preclinical drug candidates to treat schizophrenia.

Merck & Co. and Pfizer signed separate pacts for preclinical drug candidates to treat schizophrenia.

Richard Spoor, senior vice-president of global procurement at Merck & Co., Inc., discusses the company's strategy and progress made in its supply strategy that involves increased outsourcing and implementing lean-manufacturing principles in its manufacturing network. Pharmaceutical Technology's senior editor Patricia Van Arnum moderates.

Merck Sells Facility to Cherokee Pharmaceuticals, Inflazyme Announces Senior Management Resignations, More...
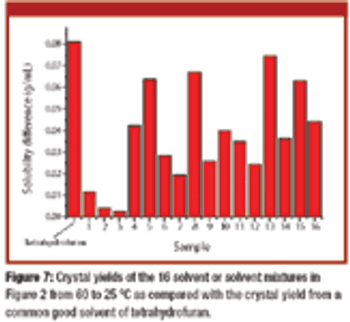
The authors propose extending initial solvent screening for a single-solvent system to the cocktail solvent screening of binary and ternary solvent mixtures.
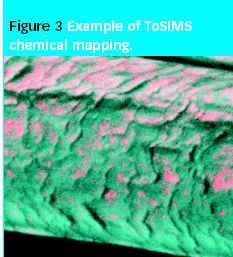
Researchers and process chemists share approaches in synthesis of active ingredients and pharmaceutical intermediates.

Brief pharmaceutical news items for January 2008.

Natural gums and mucilage are biocompatible, cheap, readily available, and represent a potential source of excipients. The authors examine the functionality of mucilage extracted from the leaves of Hibiscus rosa-sinensis Linn as an excipient in a sustained-release tablet formulation.

Pharmaceutical manufacturers often avoid putting new excipients in their formulations for fear of more regulatory oversight. IPEC has a solution.

However, companies should always be prioritizing prevention, elimination and reduction over recycling and recovery as the most effective ways of making resource efficiency savings.

Millipore and Novozymes Form Alliance, Applied Biosystems Names President and CEO, More

The US Food and Drug Administration's joint panel of the Nonprescription Drugs Advisory Committee and the Endocrinologic and Metabolic Drugs Advisory Committee voted against recommending approval of the over-the-counter use of Merck & Co.'s “Mevacor” (lovastatin) 20 mg.

Company and People Notes: West to reduce workforce, Eli Lilly's CEO and chairman to retire, more...

Company and People Notes: Eisai Acquires MGI Pharma, Ceregene Names CEO, More

Company and People Notes: Boehringer Ingelheim Expands Facility, Regulus Therapeutics Names President and CEO, More
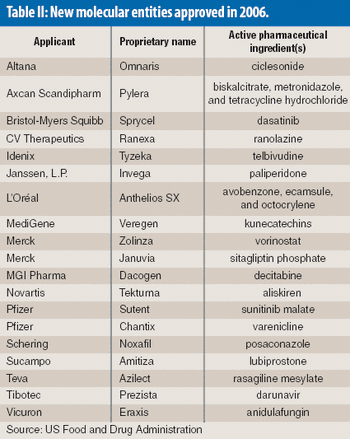
The number of new molecular entities approved by the US Food and Drug Administration is down this year as the pharmaeutical industry continues to face an innovation drought.
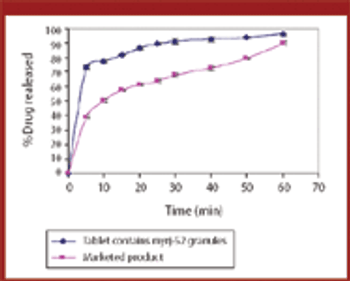
Various manufacturing techniques can improve a drug's solubility, thus increasing its bioavailability. The authors examined whether melt granulation can enhance drug solubility using meloxicam as the drug substance and myrj-52 as the binder.

A surge in capacity in contract microbial and mammalian cell-culture is underway to meet rising production needs for biopharmaceuticals.
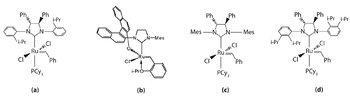
Catalysis plays a critical role in the synthesis of active pharmaceutical ingredients (APIs). It provides a way to improve yield, achieve desired stereoselectivity, improve reaction conditions, and synthesize increasingly complex APIs. Recent advances in catalyzed-olefin metathesis reveal its value and promise in the pharmaceutical industry.

In honor of Pharmaceutical Technology's 30th anniversary, the editors conducted a survey of 320 readers last spring to discuss industry advances and future directions. Here are some of your foward-looking responses.

"May you live in interesting times," goes the allegedly ancient Chinese curse. Well, these have been "interesting times" indeed for those working in the pharmaceutical industry.
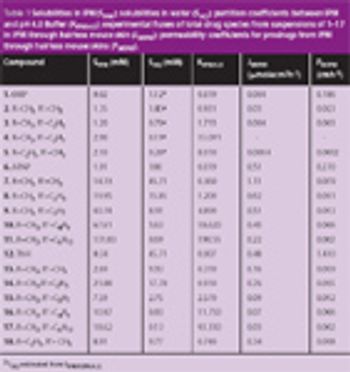
The permeation of drugs through the skin is compromised by the presence of polar functional groups such as thiols, alcohols, phenols, imides or amides. By transiently masking these polar functional groups as prodrugs the permeability of drugs containing these functional groups through the skin can be improved.

A well-devised QPP, which has been agreed on and signed by both parties, saves time and makes it easier to complete activities such as design, installations and tests.
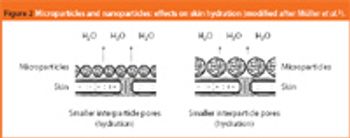
Lipid-based drug delivery systems - such as liposomes, micro-and nanoemulsions, self-emulsified drug delivery systems, and solid lipid micro-and nanoparticles - are becoming more popular because lipid materials are easily characterized, contain a high range of well-defined/tolerated surfactant molecules and can be developed for several administration routes.

Company and People Notes: ISP to raise prices, Patheon appoints CEO, more.

GlaxoSmithKline agreed to acquire Reliant Pharmaceuticals for $1.65 billion in cash.