
Pfizer's Lipitor patent revoked in Germany, promotions at Charles River Labs, more.

Pfizer's Lipitor patent revoked in Germany, promotions at Charles River Labs, more.

US Food and Drug Administration Commissioner Andrew C. von Eschenbach testified before Congress last week to outline the agency’s inspection process for foreign drug manufacturers and efforts to improve the agency’s information technology systems.

The US Food and Drug Administration’s effectiveness in regulating the manufacture of pharmaceutical products and active pharmaceutical ingredients at foreign facilities was questioned at a Congressional hearing last week. Congress, industry, and government officials weighed in on the issue.

Recent activity among contract manufacturing organizations include expansions for Lonza and Codexis in Asia and the addition of aseptic cytotoxic capabilities for DSM. Also, Vetter adds anticounterfeiting capabilities for prefilled syringes.

Ash Stevens is adding cryogenic capabilities as part of an overall strategy to focus on small molecules requiring smaller volumes and complex chemistry.

Equipped with a new sourcing strategy in Asia and improving market conditions, Pfizer CentreSource expects a good performance from its steroid business this year and in 2008.

Market demand for cytotoxic drugs is leading CMOs to expand their API manufacturing and formulation services.
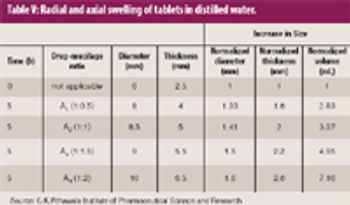
Natural gums and mucilage have been widely explored as pharmaceutical excipients. The goal of this study was to extract mucilage from the leaves of Aloe barbadensis Miller and to study its functionality as an excipient in pharmaceutical sustained-release tablet formulations.

The EIP process addresses the problems encountered with numerous questionnaires when qualifying excipient manufacturers.

The crystalline structure of pharmaceutical solids can sometimes be altered during processing. X-ray powder diffraction and near infrared spectroscopy can be used to determine the amorphous and crystalline content of a model substance. The two techniques' precision, accuracy, detection limit and the speed of analysis are compared.

Company Notes: Gibraltar expands facilities; Allozyne appoints new president and CEO, more.

After a whirlwind of negative press this fall regarding the safety of cough and cold medications for children under age 6, the US Food and Drug Administration?s Nonprescription Drugs and Pediatric Advisory Committees have recommended such over-the-counter drugs no longer be used for young children.

Company and People Notes: ImClone, BMS, and Merck form agreement; CRI Worldwide names new CEO, more.

Company and People Notes: Orexo acquires Biolipox, Avalon cofounder resigns, more...

GlaxoSmithKline and Synta Pharmaceuticals agreed to jointly develop and commercialize STA-4783. The drug is an injectable, small-molecule, oxidative stress inducer for treating metastatic melanoma that is entering Phase III clinical development.

CPhI Worldwide, the large trade show of suppliers of active pharmaceutical ingredients (APIs), intermediates, and excipients, took on a decidedly international presence.

Company and People Notes: Novartis and MIT to study continuous processing, GSK appoints Andrew Witty as CEO, more.

The European Fine Chemicals Group (EFCG) issued a position paper on excipients used in pharmaceutical manufacturing at CPhI Worldwide last week.

Company and People Notes: Thermo Fisher Scientific buys Priority Solutions International, Wyeth elects new president and CEO, more.

Identifying polymorphs is a crucial part of the drug-development process as researchers forward select methods to improve detection.

As China emerges as a significant supplier of pharmaceutical ingredients, it must assure other countries of the safety of its excipients.

A roundup of news from exhibitors at CPhI Worldwide.
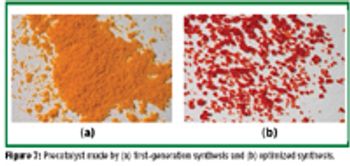
Single-enantiomer drugs represent an increasingly large share of new chemical entities, leading to approaches in asymmetric synthesis.

Amidst debate, the European Pharmacopoeia Commission is working to include physical or "functionality-related characteristics" of exipient materials in its monographs.
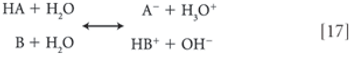
Through consideration of the ionic equilibria of acids and bases, one may readily calculate the formation constant of a salt species solely on the basis of knowledge of the pKA value of the acid and the pKB value of the base.